International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 1
ISSN 2229-5518
Venkanna Lunavath and Estari *
![]()
ABSTRACT
Human immunodeficiency virus type-1(HIV-1) is the cause of acquired immune deficiency syndrome (AIDS), a major human viral disease with about 33.2 million people infected worldwide. The current treatment for HIV/AIDS is called Highly Active Anti-Retroviral Therapy (HAART) and is a combination of anti-HIV reverse transcriptase inhibitors and protease inhibitors. The
high cost of the HAART regimen has impeded its delivery to over 90% of the HIV/AIDS population in the world. This reality has urgently called for the need to develop inexpensive alternative anti-HIV/AIDS therapy. In the current study, we characterized a panel of extracts of traditional medicinal plants for their activities against HIV-1 replication. The aim of the present study was to evaluate the invitro anti- HIV activity of Casia occidentalis plant extracts. Extracts were prepared from dried leaves in n-hexane, ethyl acetate and n butanol. Peripheral Blood Mononuclear Cells (PBMCs) isolated from healthy donors by ficoll-hypaque density gradient centrifugation method. A toxicity study was performed on all crude extracts by MTT assay using PBMCs isolated from whole blood. HIV-1 RT inhibition activity of the all solvent extracts of Casia occidentalis was determined by a RetrsoSys HIV-1 RT activity kit (Innovagen, Sweden). The leaves of Casia occidentalis extracts are shows anti- HIV-1 activity and this plant has great potential for developing useful drugs.
1 INTRODUCTION
Since the discovery of the human immunodeficiency virus as the causative agent of AIDS New chemical entities with such activity may be identified through a variety of approaches, one of them being the screening of natural products. Plant substances are especially explored due to their amazing structural diversity and their broad range of biological activities. Several plant extracts have been shown to possess activity against HIV by inhibiting various viral enzymes (Vermani et al., 2002). Various resource-poor settings, government-sponsored ART programmes discourage the use of traditional medicines, fearing that the efficacy of antiretroviral drugs may be inhibited by such natural products, or that their pharmacological interactions could lead to toxicity (Chinsembu, 2009). Medicinal plants as potential sources of new active agents not only combine the advantage of being relatively non-toxic and hence more tolerable than rationally designed drugs, but also represent an affordable and valuable source of pharmacologically active substances that can be made sufficiently available through cultivation.
With the rapid explosion of new molecular targets available for drug discovery and advances in high throughput screening technology, there has been a dramatic increase in interest from the pharmaceutical and biotechnology industries in the huge molecular diversity present in plant sources. In this study the medicinal plant extracts used in tribal areas of Warangal districts are exhibits significant potency against various bacterial and fungal pathogens, as well as potent antioxidant activity. It was therefore decided to analyse the anti-HIV activity of these potential medicinal plant and also evaluate its cytotoxicity in PBMC cell cultures. The effects of selected four medicinal plant (Phyllanthus emblica, Eclipta alba, Tinospora cordifolia and Casia occidentalis) extracts on the in vitro HIV-1 replication and cytopathic effect were determined. The ability to inhibit replication of HIV-1 could point to the potential of these four plant extracts as natural products in the chemotherapy of HIV infection.
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 2
ISSN 2229-5518
Casia occidentalis aerial parts are collected from Parvatagiri Village of Torrur Mandal, Warangal district, Andhra Pradesh. Voucher specimens were prepared and identified
at the Department of Botany, Kakatiya University, Warangal. The aerial parts of the Casia occidentalis were collected and left at room temperature for two weeks to dry, then ground into powder and extraction with soxhlet techniques with methanol. Obtaining methanolic crude extracts of Casia occidentalis were then![]() fractionated successively using solvents of increasing polarity, such as, n-hexane (HX), carbon tetrachloride (CT), and chloroform (CF) and aqueous fractions (AQ). All the four fractions (HXF, CTF, CFF and AQF) were evaporated to dryness by using rotary evaporator at low temperature (390C)
fractionated successively using solvents of increasing polarity, such as, n-hexane (HX), carbon tetrachloride (CT), and chloroform (CF) and aqueous fractions (AQ). All the four fractions (HXF, CTF, CFF and AQF) were evaporated to dryness by using rotary evaporator at low temperature (390C)
Peripheral Blood Mononuclear Cells (PBMCs) were collected from the blood of healthy volunteers, by ficol- Hypaque density gradient centrifugation method. by venipuncture and transferred into 15 ml heparin coated test tubes. The samples were diluted at 1:1 ratio with PBS, layered onto HISEP media (Himedia, Mumbai) at a volume ratio of 3:1 and centrifuged at 1,000 x g for 30 min. During the centrifugation the PBMCs moved from the plasma and were suspended in the density gradient, The PBMCs layer was removed and then washed twice with PBS.The supernatant was then removed and the cells were resuspended in RPMI 1640 medium supplemented with 1 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin, 10% inactivated FBS, and adjusted to pH 7.2 by the addition of 15 mM HEPES. The PBMC cell density used in the cytotoxicity study was 1 x 105 cells/ well of the
96-well tissue culture plate. Dose-response curves between
percentage of cell viability and concentrations of the extracts were constructed. The IC50 value was determined from the plotted curve.
Cell viability was determined by the MTT 3-(4,
5dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide)
test method. Briefly, MTT (5 mg/ml) was dissolved in PBS.
PBMC Cells were cultured in 96-well plates (1.0 x 104 cells/ well) containing 100 μl medium prior to treatment with four fractions of selected plants at 37°C for 24 h. After that,
100 μl fresh medium containing various concentrations
(0.02, 0.04, 0.09, 0.18, 0.37, 0.75 and 1.5 mg/ml) of fractional
extracts were added to each well, and incubated for another
48 h. Diluted fractional extracts solutions were freshly
prepared in DMSO, The metabolic activity of each well was
determined by the 3-(4,5 dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay and compared to those of untreated cells.
After removal of 100 μl medium, MTT dye solution was added (15 μl/ 100 μl medium) and the plates were incubated at 37°C for 4 h. After that, 100 μl of DMSO were added to each well, and mixed thoroughly. The absorbance was measured at 570 nm with a reference wavelength of
630 nm. High optical density readings corresponded to a
high intensity of dye colour that is to a high number of viable cells able to metabolize MTT salts. The fractional absorbance was calculated by the following formula
% Cell viability = Mean absorbance in test wells
![]()
X 100
Mean absorbance in control wells
The HIV reverse transcriptase enzyme inhibition due to each fraction was determined using HIV RT inhibition assay by using of RetrsoSys HIV-1 RT activity kit (Innovagen, Sweden). When determining IC50 values the substances that are to be analysed are serially diluted. The diluted substances are then added to a plate with reaction mixture. After 30 minutes of preincubation at 33°C, the reaction is started by the addition of a standardised amount of RT. The RT will now incorporate BrdUMP depending on the level of inhibition. The reaction is stopped by washing the plate. The product is quantified by the addition of the RT Product Tracer which binds to the incorporated BrdUMP. After removing excess tracer the amount of bound tracer is determined by an alkaline phosphatase / pNPP colour reaction. After correction for background signal, the measured residual RT activity for each substance dilution is calculated as a percentage of the measured RT activity in absence of inhibiting substances. Plot the percentage of residual RT activity against the concentrations of the substance dilutions for each of the tested substances. AZT (Azidothymidine/Zidovudine) was used as positive control. The inhibitory effect of each substance is expressed as an IC50 value i.e. the concentration at which 50 % of the RT activity is inhibited or the IC50 value is the substance concentration giving a 50% inhibition of the RT activity and is determined with the aid of the obtained graph. The percentage inhibition of HIV-1 RT was calculates as,
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 3
ISSN 2229-5518
Inhibition (%) = [(A control-A sample) / A control] x 100.
For statistical analysis, the results of anti-HIV-1 RT activity were expressed as means ± SD of three determinations. The IC50 values were calculated using the Microsoft Excel program. Results were considered significant if the p- values were less than 0.05.
The yield of methanol crude extract of Casia occidentalis was
38 (7.6%) g respectively. The percentage yield of these fractions of the methanolic extract of Casia occidentalis were showed in theTable-1. The HXF and CFF fractions obtained highest yield (2.5%) when compared to other fractions. 1.4% yield obtained in CTF fraction which is lowest.
Inhibition of HIV-RT by Casia occidentalis plant extract fractions were presented in Fig-1. CFF fraction of Casia occidentalis at 0.5 mg/ml concentration shows highest (88%) inhibition of HIV-RT. More than 50% inhibition of HIV-RT shows from 0.03 to 1 mg/ml concentrations of all fractions. IC50 value of CFF and AQF fractions of C. occidentalis are more than 100 mg/ml. The IC50 values of HXF and CTF fractions are 0.04 and 0.0016 mg/ml respectively are presented.
Table 1: Percentage of yield
After cells were treated with different fractions of Casia occidentalis at various concentrations for 48 h, the cytotoxic
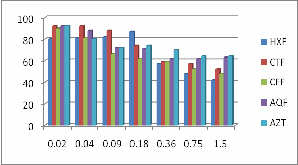
effects were investigated using the MTT assay. Cytotoxicity of each extract fraction was determined by an inhibitory concentration at 50% growth (IC50). The effect of casia occidentalis plant extract fractions on PBMC cells were presented in Fig-2. IC50 values of four fractions of C. occidentalis on PBMCs were presented in Table-15. The highest percentage of cell viability was observed in all fractions. Except CFF fraction, remaining three fractions shows 100% cell viability at 0.02 mg/ml concentration. At
1.5 mg/ml concentration all fractions of C. occidentalis shows
cytotoxic to PBMC cells.
(HXF=n-Hexane fraction, CTF=Carbon tetra chloride
fraction, CFF=Chloroform fraction, AQF=Aqueous fraction)
In this research, plants containing potential anti-HIV activity were collected and tested for HIV-1 RT inhibition.The strongest inhibitory action against HIV-1 RT was found in Casia occidentalis. The results showed that this plants contained anti-HIV properties, which was in accordance with previous reports in which the different
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 4
ISSN 2229-5518
plants A.calamus L. and P. indica L. exhibited potent antiviral activity against the Herpes simplex viruses HSV-1 and HSV-2 (Elaya Raja et al., 2009; Akanitapichat et
al.,2002). A. sativum was reported to be effective against
HIV infection by inhibiting virus replication (Harris et al.,
2001), specifically by interfering with viral reverse transcriptase activity. O. sanctum L. was found to demonstrate antibacterial, antifungal and antiviral activity (Prakash and Gupta, 2005). This report also showed that the plant possessed an anti-HIV property through inhibition of viral reverse transcriptase activity.
Experimental results thus suggested that the cassia occidentalis plant extracts which have been tested in the present study exert their anti-HIV activity via inhibition of HIV Reverse Transcriptase activity. Thus the present study down seems to justify the traditional use of plant for the treatment of infectious disease of viral origin. However, in order to assess the usefulness of this herb, it is necessary to isolate the active principle (s) from the crude and fractions, identify them and study their mechanism of action.
1) Husain A, Virmani OP, Popli SP, Misra LN, Gupta MM, Srivastava GN, Abraham Z and Singh AK, Dictionary of Indian Medicinal Plants, Central Institute of Medicinal and Aromatic Plants, Lucknow, 1992, pp. 61, 389.
2) Chinsembu KC (2009). Model and experiences of initiating collaborationwith traditional healers in validation of ethnomedicines for HIV/AIDSin Namibia. J. Ethnobio. Ethnomed. 5:30 doi:10.1186/1746-4269-5-30.
3) Wang, J.H., Tam, S.C., Huang, H., Ouyang, D.Y., Wang, Y.Y., Zheng, Y.T.,2004a. Site-directed PEGylation of trichosanthin retained its anti- HIVactivity with reduces potency in vitro. Biochemical and BiophysicalResearch Communications 317, 965–971.
4) Elaya RA, Vijayalakshmi M, Devalarao G (2009).
Acorus calamus Linn.:chemistry and biology
research. Res. J. Pharm. Technol., 2: 256–261.
5) Akanitapichat P, Kurokawa M, Tewtrakul S, Pramyothin P,Sripanidkulchai B, Shiraki K, Hattori M (2002). Inhibitory activities ofThai medicinal
plants against herpes simplex type 1, poliovirus type1, and measles virus. J. Tradit. Med., 19: 174–
180.
6) Harris JC, Cottrell SL, Plummer S, Lloyd D (2001).
Antimicrobialproperties of Allium sativum
(garlic). Appl. Microbiol. Biol., 57: 282–286.![]()
Authors: Dr. Venkanna Lunavath and Dr. Estari
Department of Zoology, Kakatiya University, Warangal-
506009. (A.P)., India
IJSER © 2012