International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 1
ISSN 2229-5518
In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics
Ankesh Kumar Srivastava, Ram Pyare and S. P. Singh
Abstract — Fe2O3 substituted 45S5 bioactive - glasses were prepared. Glass - derived Bioactive Glass - ceramics were obtained through controlled crystallization of bioactive glasses. Nucleation and crystallization regimes were determined by the parameters obtained from differential thermal analysis (DTA) of bioactive - glasses. The formed crystalline phases in bioactive glass - ceramics were identified using X - ray diffraction (XRD) analysis. Surfaces of bioactive glasses and glass - ceramics were investigated by fourier transform infrared (FTIR) reflectance spectrometry. The bioactivity of bioactive glasses and glass - ceramics was investigated through immersion studies in simulated body fluid (SBF) solution for different time periods by FTIR reflectance spectrometry with monitoring the pH changes and the concentration of silicon, sodium, calcium, phosphorus and iron ions in SBF solution. The density, micro hardness and flexural strength of bioactive glasses and glass - ceramics were measured.
Experimental results show that a decrease in glass nucleation and crystallization temperature of 45S5 bioactive - glass by doping of Fe2O3 in it and the formation of crystalline phases of sodium calcium silicate, in bioactive glass - ceramics. The bioactivity nearly remains same by doping 1% of Fe2O3 by weight, but after that it decreases. Crystalliziation of bioactive glasses decreases the bioactivity. The density, micro hardness and flexural strength of bioactive glass - ceramics are higher than their respective bioactive glasses and also it increases with increasing Fe2O3 content.
Index Terms — Bioceramics, Bioactive Glasses, Bioactive Glass - ceramics, Chemical Properties, Physical Properties, Bioactivity, Mechanical
Properties
—————————— ——————————
1 INTRODUCTION
A bioactive material is considered as the one that elicits a specific biological response at the interface that results in the formation of a bond between tissues and the materials [1]. Most of the published works on bioactive materials are concentrated on silica based glasses. Silica - based bioactive glasses have supplied successful solutions to different bone defects and soft tissue treatments during the last decades [2]. The most widely researched silica - based bioactive glasses is 45S5 bioactive glass [Composition wt. % 45 SiO2 -
24.5 Na2O - 24.5 CaO - 6 P2O5], where S denotes the network former SiO2 in 45% by weight followed by a specific Ca/P molar ratio 5 [3]. It was invented by Hench in 1969. The key compositional features that are responsible for the bioactivity of 45S5 bioactive glass are its low SiO2 (glass network former) content, high Na2O and CaO (glass network modifiers) content, and high CaO/P2O5 ratio [4]. Although 45S5 bioactive glass is biocompatible and shows high bioactivity which is in fact clinically used for middle ear prostheses and as endosseous ridge implants [5] but, it has several limitations. A major disadvantage of 45S5 bioactive glass is connected to its slow degradation rate. In addition, the mechanical properties of 45S5 bioactive glass are not completely adequate for significant load - bearing applications [6]. Previous studies [7] have shown that the partial substitution of CaO by MgO had little effect on bioactivity of this bioactive glass, while addition of 1 - 1.5 % Al2O3 by weight, prevented it. Some authors [8] argued that Table 1: Composition of bioactive glasses
the crystallization of 45S5 bioactive glass has a little effect on the ability of this bioactive glass to form a tissue bond. The aim of present investigation is to determine the bioactive behaviour, density, micro hardness and flexural strength of Fe2O3 substituted 45S5 bioactive glasses glass - ceramics.
2 EXPERIMENTAL
2.1 Preparation of Bioactive Glasses and Glass - ceramics
Fine grained quartz was used as the source of SiO2 while Na2O and CaO were introduced in the form of anhydrous sodium carbonate [Na2CO3] and anhydrous calcium carbonate [CaCO3] respectively, P2O5 was added in the form of ammonium dihydrogen orthophosphate [NH4H2PO4] and Fe2O3 was added as such for preparation of bioactive glasses. All the batch materials were of analytical grade chemicals and used without further purification. The compositions of bioactive glasses are given in Table 1. The weighed batches were melted in alumina crucibles for 3 hours in an electric furnace at the temperature 1400 ± 10 0C. The homogeneous melts were cast into preheated stainless steel moulds of the required dimensions. The prepared bioactive glass samples were directly transferred to a regulated muffle furnace at the temperature 500 0C for annealing. After 1 h, the muffle furnace was left to cool to room temperature at a rate of 30 0C/ h.
In order to obtain the bioactive glass - ceramics, the
bioactive glass samples were heated in the muffle furnace
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 2
ISSN 2229-5518


Sample

Composition (wt %)

SiO2 Na2O CaO P2O5 Fe2O3
45S5 45.00 24.50 24.50 6.00 ―
F1 44.00 24.50 24.50 6.00 1.00
F2 43.00 24.50 24.50 6.00 2.00
F3 42.00 24.50 24.50 6.00 3.00

F4 41.00 24.50 24.50 6.00 4.00

Table 2: Heat treatment schedule for crystallization of bioactive glasses
Nucleation Growth



Sample


Temperature (0C) Time (hours) Temperature (0C) Time (hours)
45S5 533 6 717 3
F1 527 6 682 3
F2 523 6 665 3
F3 519 6 650 3

F4 516 6 640 3

Table 3: Ion concentration of simulated body fluid and human blood plasma

Ion concentration (mM)

Ion Na+ K+ Mg2+ Ca2+ Cl- HCO3- HPO4- SO42-

Simulated body fluid 142.0 5.0 1.5 2.5 147.8 4.2 1.0 0.5

Human blood plasma 142.0 5.0 1.5 2.5 103.0 27.0 1.0 0.5
in two step regime at the deduced temperatures and times
as shown in Table 2. These temperatures were obtained
from differential thermal analysis (DTA) of bioactive glasses. Each bioactive glass sample was heated slowly to the first nucleation temperature for the formation of sufficient nuclei sites and after holding for the definite time, was then further heated to reach the second chosen crystal growth temperature for performing the perfect crystal growth process and after a second hold for the specific time, the sample was left to cool inside the muffle furnace to room temperature at a rate of 20 0C/h.
2.2 Physical Analysis
Differential thermal analysis (DTA) was carried out on bioactive glass samples which were examined from the temperature 300 0C up to 900 0C, using alumina as a reference material and the heating rate was 10 0C/ min. Identification of the crystalline phases after heat - treatement of bioactive glass samples was carried out by X - ray diffraction (XRD) analysis. The bioactive glass - ceramics were examined using a X - ray diffractometer, adopting Ni filter and Cu target with voltage of 40 KV and a current of 25 mA. The XRD patterns were recorded in a
2θ‖ range‖ of‖ 10‖ - 700. The JCPDS - International Center for
Diffraction Data Cards was used as a reference data for the interpretation of XRD patterns in the present work. The bioactive glass and glass - ceramic samples were
investigated by fourier transform infrared (FTIR)
reflectance spectrometry. The FTIR reflectance spectra were
obtained between wavenumber 1400 and 400 cm-1 at 2 cm-1 resolution with reference to KBr using FTIR reflectance spectrometer.
2.3 In Vitro Bioactivity Tests
In 1991, Kokubo proposed that the concept of in vitro bioactivity test which is carried out in simulated body fluid instead of living body, called in vivo bioactivity test. The ion concentration of simulated body fluid is nearly equal to that of human blood plasma and is given in Table 3 [9]. The simulated body fluid (SBF) solution was prepared by dissolving the required amounts of reagent grade chemicals, the sodium chloride [NaCl], sodium bicarbonate [NaHCO3], potassium chloride [KCl], di - potassium hydrogen phosphate [K2HPO4·3H2O], magnesium chloride hexahydrate [MgCl2·6H2O], calcium chloride dehydrate [CaCl2·2H2O] and sodium sulphate [Na2SO4] in distilled water. It was buffered at a pH value of 7.40 with 50 mM tris (hydroxymethyl) aminomethane [NH2C(CH2OH)3] and 1N
- hydrochloric [HCl] acid at the temperature 37 °C. We carried out in vitro studies by soaking polished pieces with dimension 10 mm x 10 mm x 2 mm of each bioactive glass and glass - ceramic sample in 50 ml SBF solution, at the temperature 37 °C, for 1, 3, 7 and 15 days. After soaking, the samples were filtered, rinsed with distilled water, and dried
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 3
ISSN 2229-5518
in an air oven at the temperature 150 °C for 24 hours before investigated by fourier transform infrared (FTIR) reflectance spectrometry. All the reacted SBF solution was saved for atomic absorption spectroscopic (AAS) analysis to measure ionic concentration of Si, Ca, Na, P and Fe in SBF solution. In addition, the SBF solution was also monitored for changes in pH using a pH meter before and after exposure to the SBF solution.
2.4 Density and Mechanical Properties Measurement
Archimedes principle was employed to obtain the density of bioactive glass and glass - ceramic samples using distilled water as buoyant. All the weight measurements have been made using a digital balance having an accuracy of ± 0.0001 g. Density of sample was obtained employing the relation (1) [10] as given below.

where is the weight of sample in air, is the weight of sample in buoyant and is the density of buoyant.
Micro indentations were made on the polished surfaces of
bioactive glass and glass - ceramic specimens using a diamond Vickers indenter on a micro hardness Tester. The size of the specimen was 10 mm x 10 mm x 10 mm according to ASTM Standard: C730 - 98. The indentations have been made for loads ranging between 30 mN and 2000 mN, applied at a velocity of 1 mm/s and allowed to equilibrate for 15 seconds before measurement. Micro hardness (GPa) of specimen is calculated using the formula (2) [11] as given below:

where (N) is the applied load on specimen and (m) is the diagonal of the impression.
Three-point flexural strength tests were carried out for polished bioactive glass and glass - ceramic specimens, using a universal testing machine. The size of the specimen was 4 mm x 4 mm x 50 mm according to ASTM Standard: C158 - 02. The load was applied over a 40 mm span and at the mid - point of the 4 mm x 40 mm surface using a cross - head speed of 0.5 mm/min. Flexural Strength of specimen is calculated using the formula (3) [12] as given below:

where is the load at which specimen being fractured, is the length of specimen over which the load is applied, is the width of specimen, and is the height of specimen.
3 RESULTS
3.1 Physical Analysis
3.1.1 Differential Thermal Analysis (DTA)
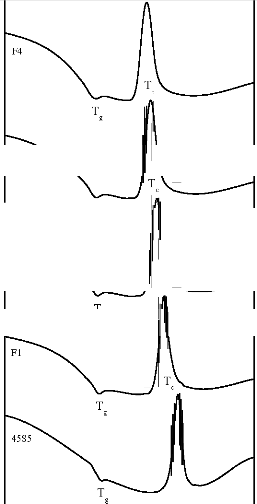
The Differential Thermal Analysis (DTA) traces of bioactive glasses are shown in Figure 1. The DTA traces of bioactive glasses show that the incorporation of Fe2O3 in the base bioactive glass (45S5) causes a decrease in its endothermic peak temperature as well as its exothermic peak temperature.
3.1.2 X - Ray Diffraction (XRD) Analysis
The X - ray diffraction (XRD) patterns for bioactive glass - ceramics are shown in Figure 2. The XRD patterns of all the bioactive glass - ceramics show the presence of crystalline phase of sodium calcium silicate [Na2Ca2 Si3O9 (card number: PDF # 01 - 1078 & PDF # 02 - 0961), Na2CaSi3O8 (card number: PDF # 12 - 0671)].
3.1.3 Fourier Transform Infrared (FTIR) Reflectance
Spectrometric Investigation
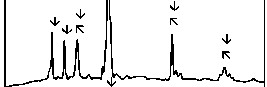
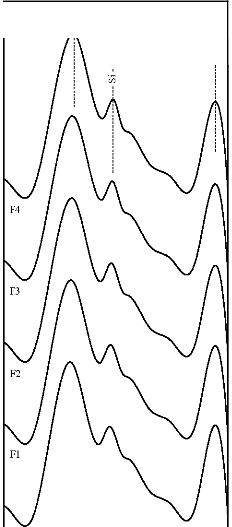
The fourier transform infrared (FTIR) reflectance spectra of bioactive glasses and glass - ceramics before immersion in simulated body fluid (SBF) solution are shown in Figure 3. The FTIR reflectance spectra of bioactive glass 45S5 reveals sharp peaks at wavenumbers 471, 930 and 1100 cm-1 while its glass - ceramic shows additional peaks at wavenumbers
580, 650 and 1041 cm-1. The FTIR reflectance spectra of each Fe2O3 substituted bioactive glasses (F1, F2, F3, and F4) and their glass - ceramics seems to be repetitive to that obtained from the base bioactive glass (45S5) and its glass - ceramic respectively.
3.2 In Vitro Bioactivity Tests
3.2.1 Fourier Transform Infrared (FTIR) Reflectance
Spectrometric Investigation
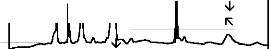
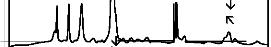
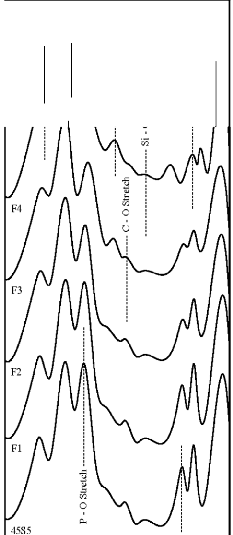
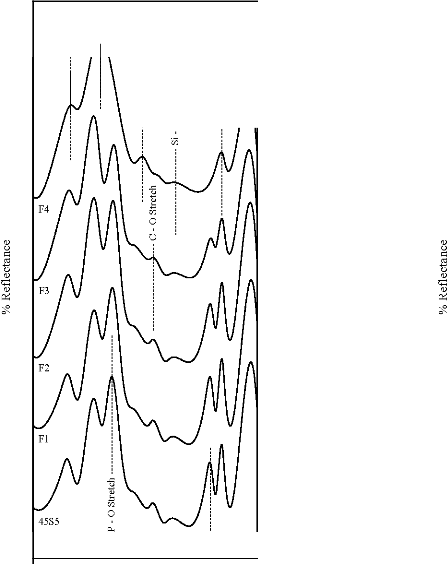
The fourier transform infrared (FTIR) reflectance spectra of bioactive glasses and glass - ceramics after soaking in simulated body fluid (SBF) solution for a period of 1, 3, 7, and 15 days are given in Figures 4, 5, 6 and7 respectively. Following changes were observed in the FTIR reflectance spectra of bioactive glass 45S5 at various reaction times. After soaking for 1 day in SBF solution peak at wavenumber 471 cm-1 shifted to lower wavenumber at 461 cm-1 and peak at wavenumber 1100 cm-1 shifted to higher wavenumber at 1125 cm-1 with decreasing their intensity, while the peak at wavenumber 930 cm-1 had disappeared. Appearance of new peaks at wavenumbers 557, 607, 794,
871, 1050, and 1250 cm-1 were observed. After 3 days intensity of peaks at wavenumbers 557, 794, 1125, 1250 cm-1 decreased while the intensity of peaks at wavenumbers 607,
871, 1050 cm-1 increased. After 7 days peak at wavenumber
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, Februa ry-2012 4
ISSN 2229-5518


T
c
T F4
..v
..v
·.a...
(!)
il T
'"". " ':><
;; w
F3
::i ..v
'-../ '-../
:>-,
0 .';:::
<;::1 e.:n::
0 'l)
(!)
::r:: ....
..c::
.8 F2
.::: ..v ..v
..v

..v ..v

Fl ..v
..v
..v

..v ..v

300 400 500 600 700 800 900
Temperature (°C)

45S5
10 20
30 40
28 (degree)
50 60 70
Tg = Glass Nucleation Temperature
[loij Na Ca Sip
["'l Na Casip
T, = Glass Crystallization Temperature
Figure 1:
2 2 9 2 8
DTA traces of bioactive glasses
Figure 2: XRD patterns of bioactive glass - ceramics
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 5
ISSN 2229-5518
>:Q
Si - 0 - Si Stretch I Asynun
..c::
Of.)
0
Si - 0 - Si Bend
Si - 0 - Si Stretch I Asynun
] Si-0-SiBend
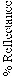
0
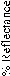
45S5
45S5

1400
1200 1000 800
Wavenumber (em-1
(A)
600 400
1400
1200 1000 800
Wavenumber (em-1
(B)
600 400
Figure 3: FTIR reflectance spectra of (A) bioactive glasses (B) bioactive glass- ceramics before immersion in SBF solution
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 6
ISSN 2229-5518
Si- 0- Si Stretch / Asymm Si - 0 - Si Bend Si- 0- Si Stretch I Asymm
a ..<::
Si- 0 - Si Bend
..<::
f!l
<i "'
.i:l "'
u tZl
<i
tZl
tZl
0
.i:l ..<:: ..<:: "0 "0 .!:l 0::
tZl
<i 0:: " "
0 .i:l .i:l
tZl tZl 0
0/i 0/i 0 0/i
0 p.. 0
p..
P - 0 Bend I Crystal
P - 0 Bend I Crystal

1400
1200 1000 800
Wavenumber (cm-1
(A)
600 400
1400
1200 1000 800
Wavenumber (cm-1
(B)
600 400
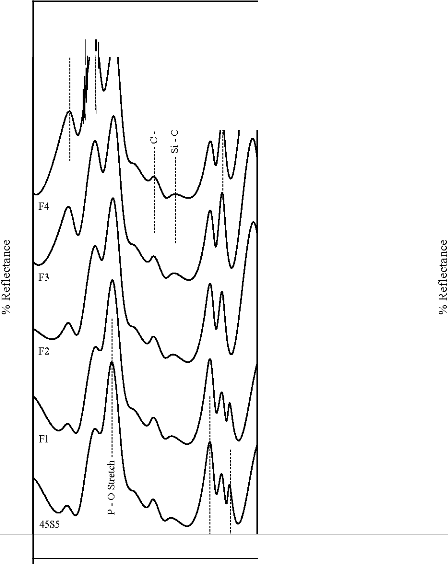
Figure 4: FTIR reflectance spectra of (A) bioactive glasses (B) bioactive glass- ceramics after soaking for a period of 1 day in SBF
solution
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 7
ISSN 2229-5518
Si- 0- Si Stretch / Asymm Si- 0- Si Bend Si- 0- Si Stretch / Asymm Si- 0- Si Bend
a 6 (.) f!l
-o "1:l a 6"'
.c:
(/)
0::: (/)
" 0 -o
(.) .c: (/) 0:::
ti (.) 0 "
1:l " .c:
(/) 1:l p.. .c: .!:l 0
(/)
·u:;
"1:l
1":l
(/)
p..
u (/) <ii
0 0
P - 0 Bend I Qystal
P - 0 Bend I Crystal

1400
1200 1000 800
Wavenumber (cm-1
(A)
600 400
1400
1200 1000 800
Wavenumber (cm-1
(B)
600 400
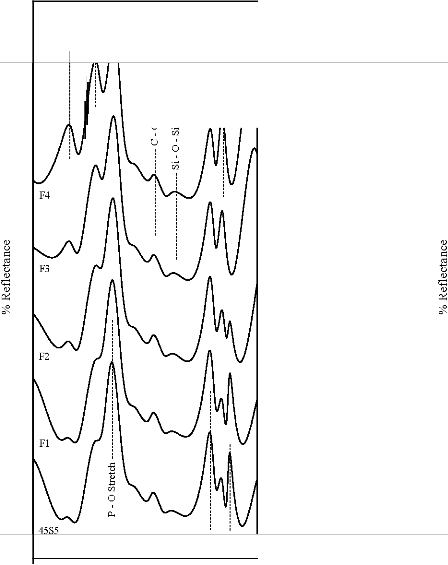
Figure 5: FTIR reflectance spectra of (A) bioactive glasses (B) bioactive glass- ceramics after soaking for a period of 3 days in SBF
solution
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 8
ISSN 2229-5518
Si- 0- Si Stretch / Asymm Si - 0 - Si Bend S1- 0- S1 Stretch / Asymm Si- 0- Si Bend
g: rn
"' rn
E 6
-o E
6"'
-o
(/) 0::
p::) (/)
p"::)
..<::
'B 0
-5 ..<:: 0
" 1":::
p, " "
1::: p,
1::: (/) (/)
(/)
(/) 0 <ii
u 0
P - 0 Bend I Crystal
P - 0 Bend I Crystal

1400
1200 1000 800
Wavenumber (em-1
600 400
1400
1200 1000 800
Wavenumber (em-1
600 400
(A)
(B)
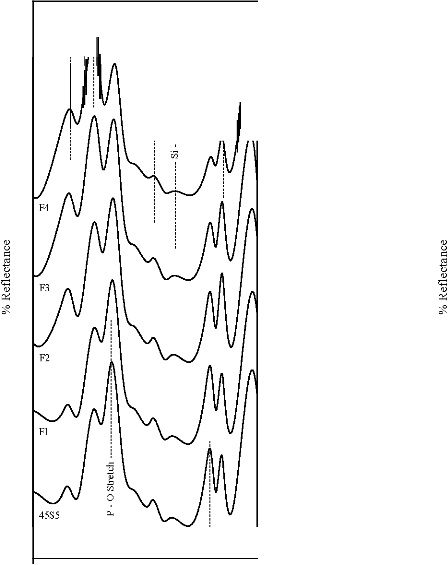
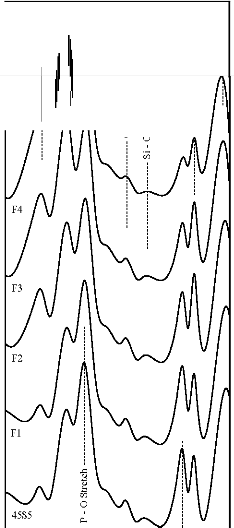
Figure 6: FTIR reflectance spectra of (A) bioactive glasses (B) bioactive glass- ceramics after soaking for a period of 7 days in SBF
solution
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 9
ISSN 2229-5518
S1- 0- S1 Stretch / Asymm Si- 0- SiBend Si- 0- Si Stretch / Asymm Si- 0- Si Bend
ill
ill "'
6"' a 6
a -o <ll
-o
0::
<ll
0:: "
p::) u 0
0 _c::
u _c:: .':l
t>
.b
<ll p,
"'ii .':l ."b <Zi
.b " <ll
0 <ll 0
P - 0 Bend I Crystal P - 0 Bend I Crystal

1400
1200 1000 800
Wavenumber (em- 1
600
400
1400 1200 1000 800
Wavenumber (em-1
600 400
(A)
(B)
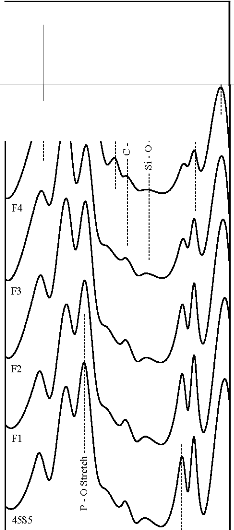
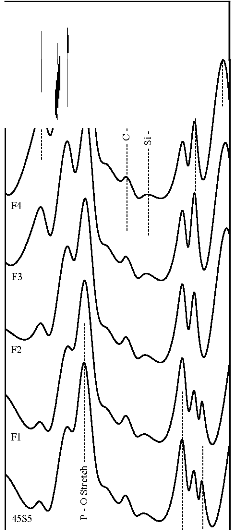
Figure 7: FTIR reflectance spectra of (A) bioactive glasses (B) bioactive glass- ceramics after soaking for a period of 15 days in
SBF solution
IJSER 2012 http /fwww.llser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 10
ISSN 2229-5518

Table 4: Density, micro hardness and flexural strength of bioactive glasses and glass - ceramics
Density (g/cm3) Micro hardness (GPa) Flexural strength (MPa)




Sample



Glasses Glass - ceramics Glasses Glass - ceramics Glasses Glass - ceramics
45S5 2.707 2.912 5.75 7.70 43.48 104.17
F1 2.723 2.925 5.91 7.82 53.25 113.47
F2 2.731 2.932 5.98 7.88 60.42 119.20
F3 2.746 2.946 6.11 8.00 69.52 127.32

F4 2.757 2.956 6.20 8.08 77.33 134.46
471 cm-1 has founded disappear. Appearance of peak at
wavenumbers 527 cm-1 was observed. After 15 days, peaks
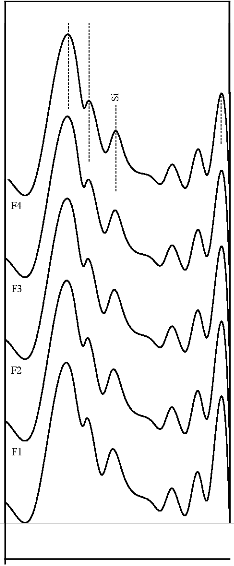
at wavenumbers 527, 607, 871, 1050 cm-1 were dominant in the FTIR reflectance spectra. Careful inspection of FTIR reflectance spectra of all the Fe2O3 substituted bioactive glasses (F1, F2, F3, and F4) in comparison with the base bioactive glass (45S5) reveals minor or limited variation of the positions and intensities of the reflectance peaks. The main differences can be summarized in bioactive glasses, where there was time delay in the formation of peaks at wavenumbers 527 and 607 cm-1. After soaking for 15 days in SBF solution (Figure 7A) it was found that the intensity of peak at these wavenumbers nearly remains same by doping of 1% Fe2O3 by weight with respect to parent bioactive glass (45S5), but afterwards as well as Fe2O3 content increases a decrease in intensity was observed. The FTIR reflectance spectra of bioactive glasses and glass - ceramics after soaking for 15 days in SBF solution (Figure 7A and 7B respectively) shows that peaks at wavenumbers 527 and
607 cm-1 was found less intense in the bioactive glass - ceramics than their respective bioactive glasses.
3.2.2 Ion Release Analysis
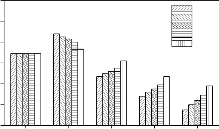
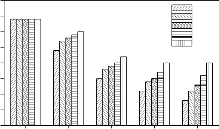
Variations of Si, Na, Ca, P and Fe concentration in simulated body fluid (SBF) solution due to soaking of bioactive glasses and glasses - ceramics for various time periods are shown in Figure 8. As can be observed in all cases that Si concentration in SBF solution increased during first 7 days of soaking and then a slight decrease was obtained. Na concentration increased rapidly during first 3 days of soaking and then it attains nearly a constant value where as Ca concentration increased during first day of soaking and then it decreased continuously. Increase in Fe concentration and a decrease in P concentration were also observed. It was also observed that the addition of Fe2O3 in the base bioactive glass (45S5) decreases the leaching rate of ions and crystalliziation of bioactive glasses also decreases the leaching rate of ions.
3.2.3 pH Measurements
The variation in pH values of simulated body fluid (SBF)
solution due to soaking of bioactive glasses and glass -
ceramics for various time periods is shown in Figure 9. The
pH Value of SBF solution increased during first 3 days of
soaking and then it attained nearly a constant value in all cases. . It was also observed that the addition of Fe2O3 in the base bioactive glass (45S5) decreases the pH value and crystallizing the bioactive glasses also decreases pH value of SBF solution.
3.3 Density and Mechanical Properties Measurement
Experimental values of density, micro hardness and flexural strength of bioactive glasses and glass ceramics are given in Table 4. It has been observed that the increase of Fe2O3 in the base bioactive glass (45S5) causes an increase in its density, micro hardness and flexural strength. It also has been observed that the density, micro hardness and flexural strength of bioactive glass - ceramics are higher than their respective bioactive glasses.
4 DISCUSSIONS
4.1 Physical Analysis
4.1.1 Differential Thermal Analysis (DTA)
In the differential thermal analysis (DTA) traces of bioactive glasses (Figure 1) endothermic peak show the nucleation region and the exothermic peak corresponding to the crystallisation process. Previous studies [13] have shown that in silicate glasses, the presence of transition metal ions in low doping percent is not expected to form separate structural units. It can be assumed that the transition metal ions in low level behave as a network modifier. A decrease of glass nucleation and crystallization temperature of the base bioactive glass (45S5) with the addition of Fe2O3 can be related to lower structural bonding [14].
4.1.2 X – Ray Differaction (XRD) Analysis
The XRD patterns of all the bioactive glass - ceramics show the presence of crystalline phases. The reason for the ease of crystallization of bioactive glasses can be correlated with the presence of silicate and phosphate network, as well as the possible phase separation even in micro scale of the two phases on heat - treatment. It is well known that the addition of a few percentage of P2O5 to silicate glass compositions, promotes the volume nucleation and glass - ceramic formation [15]. There is some evidence for
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 11

ISSN 2229-5518

120
100
80
45S5
F1
F2
F3
F4
Glasses
120
100
80
45S5
F1
F2
F3
F4
Glass - ceramics
60 60
40 40
20 20
0
day 0 day 1 day 3 day 7 day 15

Time
0
day 0 day 1 day 3 day 7 day 15

Time
3700
3600
3500
45S5
F1
F2
F3
F4
Glasses
3700
3600
3500
45S5
F1
F2
F3
F4
Glass - ceramics
3400
3400
3300
3300
3200
3200
3100
day 0 day 1 day 3 day 7 day 15

Time
3100
day 0 day 1 day 3 day 7 day 15
Time
140
120
100
Glasses
45S5
F1
F2
F2
F3
140
120
100
Glass - ceramics
45S5
F1
F2
F3
F4
80 80
60 60
40 40
20
day 0 day 1 day 3 day 7 day 15
Time
20
day 0 day 1 day 3 day 7 day 15
Time

40
Glasses
35
30
25
45S5
F1
F2
F3
F4
40
Glass - ceramics
35
30
25
45S5
F1
F2
F3
F4
20 20
15 15
10 10
5 5
0
day 0 day 1 day 3 day 7 day 15

Time
0
day 0 day 1 day 3 day 7 day 15

Time
4.0
3.5
3.0
2.5
45S5
F1
F2
F3
F4
Glasses
4.0
3.5
3.0
2.5
45S5
F1
F2
F3
F4
Glass - ceramics
2.0
2.0
1.5
1.5
1.0
1.0
0.5
0.5
0.0
day 0 day 1 day 3 day 7 day 15
Time
0.0
day 0 day 1 day 3 day 7 day 15
Time
Figure 8: Variation in Si, Na, Ca, P and Fe concentration in SBF solution due to soaking for various time periods
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 12
ISSN 2229-5518
11.0
10.5
10.0
9.5
45S5
F1
F2
F3
F4
Glasses
11.0
10.5
10.0
9.5
45S5
F1
F2
F3
F4
Glass - ceramics
9.0
9.0
8.5
8.5
8.0
8.0
7.5
7.5
7.0

day 0 day 1 day 3 day 7 day 15
Time
7.0

day 0 day 1 day 3 day 7 day 15
Time
Figure 10: Variation in pH of SBF solution due to soaking for various time periods
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 13
ISSN 2229-5518
precipitation of phosphate crystals which subsequently act
as heterogeneous nucleation sites for the subsequent crystallization of the major phases, although the detailed role of P2O5 remains to be discussed [16]. Previous studies [17], [18], [19] have shown that the heat - treatment of 45S5 bioactive glass at a nucleation temperature of 550 0C and followed by heating at a crystallization temperature of 680
0C produces a bioactive glass - ceramic containing the sodium calcium silicate [Na2Ca2Si3O9] as main crystallline phase. In all the bioactive glass - ceramics sodium calcium silicate [Na2Ca2 Si3O9 & Na2CaSi3O8] is present as main crystalline phase. The studied bioactive glass - ceramics did not contain Fe as separate crystalline phases. This can be related to their relatively low content in bioactive glass composition.
4.1.3 Fourier Transform Infrared (FTIR) Reflectance
Spectrometric Investigation
The fourier transform infrared (FTIR) reflectance spectra of bioactive glasses and glass - ceramics before immersion in simulated body fluid (SBF) solution (Figure 3) reveal Si - O - Si bending (500 - 400 cm-1), Si - O stretching (940 - 860 cm-1) and Si - O - Si stretching (asymmetric) (1200 - 970 cm-1) bands, which are known and accepted to be mainly characteristic of silicate network [20], [21], [22]. This may be attributed due to the presence of major SiO2 as a basic building constituent. The FTIR reflectance spectra of bioactive glasses and glass - ceramics did not show separate bands due to the presence of phosphate network and this may be due to the limited percentage of P2O5. The FTIR reflectance spectra of bioactive glass - ceramics also, show the additional bands at wavenumbers 650 - 619 cm-1 and
580 - 570 cm-1 which are due to presence of sodium calcium silicate [Na2Ca2 Si3O9] crystalline phase [23].
4.2 In Vitro Bioactivity Tests
4.2.1 Fourier Transform Infrared (FTIR) Reflectance
Spectrometric Investigation
The fourier transform infrared (FTIR) reflectance spectra of bioactive glasses and glass - ceramics after soaking in simulated body fluid (SBF) solution for different times (Figures 4, 5, 6 and 7) reveal Si - O - Si stretching (symmetric) (820 - 770 cm-1) and (asymmetric) (1200 - 970 cm-1) bands, which indicates the formation of silica - rich layer. The presence of P - O bending (amorphous) (560 - 550 cm-1) bands indicates the formation of CaO - P2O5 layer. Emerging of P - O bending (crystalline) (610 - 600 cm-1 and (530 - 515 cm-1) bands indicates the formation of hydroxyl carbonate apatite (HCA) layer. Presence of C - O stretching (890 - 800 cm-1) bands shows the crystalline nature of HCA layer and P - O stretching (1040 - 910 cm-1) bands are attributed due to HCA layer [24], [25], [26]. Intensity of
silica - rich layer and CaO - P2O5 layer goes on decreasing but intensity of HCA layer increases with time in all cases
after soaking for 1 day in SBF solution. Hench et.al. were
the first to detail a number of sequential steps in the in vitro and in vivo reactivity of silicate glasses that are responsible for the tissue bonding ability of these glasses. Briefly, these involve cation release from the glass with consequential increase in pH of solution, formation of silica - rich layer and precipitation of a CaO - P2O5 rich layer that further crystallizes as HCA layer [27], [28], [29].The degree of bioactivity in bioactive material is usually expressed by the formation of HCA surface. Finally, the FTIR reflectance spectra of bioactive glasses after soaking for 15 days in SBF solution (Figure 7A) indicates that addition of more than
1% of Fe2O3 by weight in the base bioactive glass (45S5) decreases its bioactivity.. This is because of the fact that transition metals enhance the chemical durability of silicate glasses [30]. The FTIR reflectance spectra of bioactive glasses and glass - ceramics after soaking for 15 days in SBF solution (Figure 7A and 7B) shows that the bioactivities of bioactive glass - ceramics are less than their respective bioactive glasses. This phenomenon is explained by considering that the amorphous phase is usually more prone to ion leaching phenomena than crystalline phases [31]. Therefore, the suppression of the formation of silica - rich layer leads to the suppression of CaO - P2O5 layer and hence suppression of the formation of HCA surface.
4.2.2 Ion Release Analysis
The quantitative determination of Si, Na, Ca, P and Fe ions in simulated body fluid (SBF) solution for various times (Figures 8 ) is important to understand the kinetics of surface reactions in bioactive glasses and glass - ceramics. The decrease in Ca and P concentrations with a simultaneous increase in Si concentrations is consistent with the formation of CaO - P2O5 layer. The participation of Fe in the nucleation process can be ascertained by the observed variation in its concentration with soaking time.
4.2.3 pH Measurements
During initial period of soaking, faster release of Ca and Na ions increased the pH value, but after that pH attained nearly a constant value since rate of release of Na ion decreased (Figure 9).
4.3 Density and Mechanical Properties Measurement
The increase of Fe2O3 in the base bioactive glass (45S5) leads to an increase its density because of replacement of a lighter element, Si (density = 2.33 g/cm3) with a heavier element, Fe (density = 7.86 g/cm3). The increase of Fe2O3 in the base bioactive glass (45S5), also leads to an increase its micro hardness and flexural strength. This is easily understood that the more the density of glass, the more the compactness of glass structure, and consequently, the more micro hardness and flexural strength. The density, micro hardness and flexural strength of bioactive glass - ceramics
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 14
ISSN 2229-5518
are higher than their respective bioactive glasses due to
densification.
5 CONCLUSIONS
In the present investigation, a comparative study was made on physical, bioactive and mechanical properties of Fe2O3 substituted 45S5 bioactive glasses and glass - ceramics. The following conclusions are obtained from this investigation: [1] Increasing the Fe2O3 content in 45S5 bioactive glass decreases its glass nucleation and crystallization temperature. There is no effect on bioactivity by doping of
1% of Fe2O3 by weight in 45S5 bioactive glass but
afterwards it goes on decreasing successively. Increasing the Fe2O3 content in 45S5 bioactive glass enhances its chemical durability, density, micro hardness and flexural strength.
[2] Crystallization of bioactive glasses decreases their
bioactivity but increases their chemical durability, density, micro hardness and flexural strength.
References
[1] L.L. Hench and O. Andersson, “An Introduction to Bioceramics”,‖ editors,‖ L.L.‖ Hench‖ and J. Wilson, World Scientific, Singapore, pp. - 41, 1993.
[2] D. Arcos and M. Vallet - Regí, “Sol - gel silica - based
biomaterials and bone tissue regeneration”, Acta
Biomaterialia, vol. 6, pp. 2874 - 2888, 2010.
[3] S.M. Best, A.E. Porter, E.S. Thian and J. Huang, “Bioceramics: Past,‖ present‖ and‖ for‖ the‖ future“,‖ Journal of the European Ceramic Society, vol. 28, pp. 1319 - 1327, 2008. [4] M.N. Rahaman, D.E. Day, B.S. Bal, Q. Fu, S.B. Jung, L.F. Bonewald and A.P. Tomsia, “Bioactive glass in tissue engineering”,‖ Acta‖ Biomaterialia,‖ vol. 7, pp. 2355 - 2373,
2011.
[5] C.C. Lin, L.C. Huang and P. Shen, “Na2CaSi2O6 - P2O5
based bioactive glasses. Part 1: Elasticity and structure”, Journal of Non - Crystalline Solids, vol. 351, pp. 3195 - 3203,
2005.
[6] V. Aina, G. Malavasi, A.F. Pla, L. Munaron and C. Morterra, “Zinc - containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells”,‖ Acta‖ Biomaterialia, vol. 5, pp. 1211 - 1222, 2009.
[7] G. Lusvardi, G. Malavasi, L. Menabue, V. Aina and C. Morterra, “Fluoride - containing bioactive glasses: Surface reactivity in simulated body fluids solutions”,‖ Acta‖ Biomaterialia, vol. 5, pp. 3548 - 3562, 2009.
[8] D.C. Clupper, J.J. Mecholsky Jr., G.P. LaTorre and D.C. Greenspan, “Sintering temperature effects on the in vitro bioactive response of tape cast and sintered bioactive glass - ceramic in Tris buffer”, Journal of Biomedical Materials Research, vol. 57, pp. 532 - 540, 2001.
[9] T. Kokubo and H. Takadama, “How useful is SBF in
predicting in vivo bone bioactivity?”,‖ Biomaterials,‖ vol. 27, pp. 2907 - 2915, 2006.
[10] V. Rajendran, A.N. Begum, M.A. Azooz and F.H.
ElBatal, “Microstructural dependence on relevant physical
- mechanical properties on SiO2 - Na2O - CaO - P2O5
biological glasses”, Biomaterials, vol. 23, pp. 4263 - 4275,
2002.
[11] M.D. Michel, A. Mikowski, C.M. Lepienski, C.E.
Foerster and F.C. Serbena, “High temperature microhardness of soda - lime glass”, Journal of Non - Crystalline Solids, vol. 348, pp. 131 - 138, 2004.
[12] Q.Z. Chen, I.D. Thompson and A.R. Boccaccini, “45S5
Bioglasss - derived glass - ceramic scaffolds for bone tissue
engineering”, Biomaterials, vol. 27, pp. 2414 - 2425, 2006. [13] E.M.A. Khalil, F.H. ElBatal, Y.M. Hamdy, H.M. Zidan, M.S. Aziz and A.M. Abdelghany, “Infrared absorption spectra of transition metals - doped soda lime silica glasses”,‖Physica‖B,‖vol. 405, pp. 1294 - 1300, 2010.
[14] A. Agarwal and M. Tomozawa, ”Correlation of silica glass properties with the infrared spectra”, Journal of Non - Crystalline Solids, vol. 209, pp. 166 - 174, 1997.
[15] F.H. ElBatal and A. ElKheshen, “Preparation and characterization of some substituted bioglasses and their ceramic derivatives from the system SiO2 - Na2O - CaO - P2O5 and effect of gamma irradiation”, Materials Chemistry and Physics, vol. 110, pp. 352 - 362, 2008.
[16] P.F. James, “Glass ceramics: new compositions and uses”, Journal of Non - Crystalline Solids, vol. 181, pp. 1 -
15, 1995.
[17] L.L. Hench, R.J. Splinter, T.K. Greenlee and W.C. Allen, “Bonding mechanisms at the interface of ceramic prosthetic materials”, Journal of Biomedical Materials Research, vol. 2 (part 1), pp. 117 - 141, 1971.
[18] L.L. Hench and H.A. Paschall, “Direct chemical bonding of bioactive glass - ceramic materials and bone”, Journal of Biomedical Materials Research: Symp., vol. 4, pp.
25 - 42, 1973.
[19] V.R. Mastelaro, E.D. Zanotto, N. Lequeux and R. Cortes, “Relationship between short - range order and ease of nucleation in Na2Ca2Si3O9, CaSiO3 and PbSiO3 glasses”, Journal of Non - Crystalline Solids, vol. 262, pp. 191 - 199,
2000.
[20] J. Serra, P. Gonzalez, S. Liste, S. Chiussi, B. Leon and M. Perezamor, “Influence of the non - bridging oxygen groups on the bioactivity of silicate glasses”, Journal of Materials science : Materials in medicine, vol. 13, pp. 1221 - 1225,
2002.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about In Vitro Bioactivity and Physical - Mechanical Properties of Fe2O3 substituted 45S5 Bioactive Glasses and Glass - ceramics 15
ISSN 2229-5518
[21] J. Marchi, D.S. Morais, J. Schneider, J.C. Bressiani and
A.H.A. Bressiani, “Characterization of rare earth aluminosilicate‖glasses”,‖Journal of Non - Crystalline Solids, vol. 351, pp. 863 - 868, 2005.
[22] M. Wang, J. Cheng, M. Li and F. He, “Structure and properties of soda lime silicate glass doped with rare earth”,‖Physica‖B,‖vol.‖406, pp. 187 - 191, 2011.
[23] H.A. ElBatal, M.A. Azooz, E.M.A. Khalil, A.S. Monem and Y.M. Hamdy, “Characterization of some bioglass - ceramics”, Materials Chemistry and Physics, vol. 80, pp. 599
- 609, 2003.
[24] M. R. Filgueiras, G. P. LaTorre and L. L. Hench, “Solution effects on the surface reactions of three bioactive glass compositions”, Journal of Biomedical Materials Research, vol. 27, pp. 1485 - 1493, 1993.
[25] O.P. Filho, G.P. LaTorre and L.L. Hench, “Effect of crystallization on apatite layer formation of bioactive glass
45S5”, Journal of Biomedical Materials Research, vol. 30, pp. 509 - 514, 1996.
[26] O. Peitl, E.D. Zanotto and L.L. Hench, “Highly bioactive P2O5 - Na2O - CaO - SiO2 glass - ceramics”, Journal of Non - Crystalline Solids, vol. 292, pp. 115 - 126, 2001.
[27] L.L. Hench, “Bioceramics: From Concept to Clinic”, Journal of the American Ceramic Society, vol. 74, pp. 1487 -
1510, 1991.
[28] F. Branda, R. Fresa, A. Costantini and A. Buri, “Bioactivity of 1.25 CaO - SiO2 glass: an FTIR and X - Ray study on powered samples”,‖ Biomaterials,‖ vol. 17, pp. 2247
- 2251, 1996.
[29] A. Balamurugan, G. Balossier, S. Kannan, J. Michel, A.H.S. Rebelo and J.M.F. Ferreira, “Development and in vitro characterization of sol - gel derived CaO - P2O5 - SiO2 - ZnO bioglass”,‖ Acta‖ Biomaterialia,‖ vol. 3, pp. 255 - 262,
2007.
[30] F.H. ElBatal, E.M. Khalil,Y.M. Hamdy, H.M. Zidan, M.S. Aziz and A.M. Abdelghany, “FTIR Spectral Analysis of Corrosion Mechanisms in Soda Lime Silica Glasses Doped with Transition Metal Oxides”,‖Silicon,‖vol. 2, pp. 41
- 47, 2010.
[31] E. Verne, O. Bretcanu, C. Balagna, C.L. Bianchi, M.
Cannas, S. Gatti and C. Vitale Brovarone, “Early stage reactivity and in vitro behavior of silica - based bioactive glasses and glass - ceramics”, Journal of Materials Science: Materials in Medicine, vol. 20, pp. 75 - 87, 2009.
————————————————
■ Ankesh Kumar Srivastava, Ram Pyare and S. P. Singh
Department of Ceramic Engineering, Institute of Technology
Banaras Hindu University, Varanasi - 221005: INDIA
■ Corresponding Author
Ankesh Kumar Srivastava
E - mail: ankesh.000@gmail.com
IJSER © 2012 http://www.ijser.org
























