International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1840
ISSN 2229-5518
Impact Assessment Of Industrial Waste On Groundwater & Its Remediation: A Case Study Of Hexavalent Chromium Contamination Of Lohiya Nagar, Ghaziabad
S.K.Singh, Dr. Gauhar Mahmood and Dr. Divya Chinche
This research paper deals with the management of groundwater contamination of lohia nagar Ghaziabad caused by hexavalent chromium. According to sources large amount of chromium is seeped into the groundwater and affected the water supply at many locations it is being noted that the quantity of chromium was six times the maximum acceptable limits as permissible in drinking water. The carcinogenic effects of hexavalent chromium are very well known by scientists and officials and which should be dealt with as early as possible. This study was conducted in area is located in Lohiya Nagar of Ghaziabad district. The area comprises of a number of the industries of various nature including plates, electroplating, various mechanical manufacturing units, etc. The same time the area has got residential pockets also. The impact of the groundwater contamination occurs in almost the entire part of the study area except few localized pockets. About 21 sounding stations and 20 water sampling stations have been chosen on the basis of hydrogeological spectrum of the area. A comprehensive study of the area is done in which qualitative and quantitative study of area is done i.e. groundwater resource potential etc is studied. It is found that contamination of chromium (Hexavalent) is highest in north-western part of the study area which is more than 1.3 mg/l and the permissible limit is 0.05. The analysis further shows that the 02 Trough in the area has been recorded which are as follows: First through in between Dewan Rubber and Muscat, Second through at Banke Bihari Temple. These troughs are converted into plumes of hexavalent chromium and the concentration level becomes high due to natural trough formation supported by low permeable and high porous strata. The cross sections of these plume areas further indicated that at about 190 – 200 feet the concentrations of the hexavalent chromium high to medium. However, the concentration level of this plumed chromium reduces with depth. Evidently, in the pumping station of the Lohiya Nagar Pump House, the concentration of the hexavalent chromium is within permissible limits. There are several mitigation measures adopted to remove the contamination of chromium from this are, out of several different techniques Bioremediation Technique is selected for the said process. Complete removal of hexavalent chromium will take several years.
Keywords- Bioremediation, Electroplating, Groundwater Remediation, Hexavalent Chromium, Heavy metal, Oxidation- reduction potential
W astewater,
————————————————
• S.K.SINGH, Uttar Pradesh Pollution Control Board, Lucknow
• DR. GAUHAR MAHMOOD , Professor Jamia Millia Islamia, New Delhi-
110025), E.Mail: aquaexplorers@yahoo.com
• DR. DIVYA CHINCHE, Research Analyst, Jamia Millia Islamia-
110025), E.Mail: divya.khale@gmail.com
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1841
ISSN 2229-5518
In today's industrial society, matters are, among the most commonly used raw material. Mining, metal refining use of metals in manufacturing and the final disposition of manufactured product constitute activities resulting in metal loses. Metal wastes represents a critical loss of non renewable resources and pose serious health and ecological risk. Industrial waste has been poorly managed and is becoming a major problem in industrialized regions. Agriculture, chemical, textile and metallurgic industries consume large amounts of water that are released into the environment after processing and contain dissolved toxic substances such as acids, bases, toxic chemical compounds and heavy metals, all potentially harmful to the environment [1].
International agreements and directives issued
by various countries prohibit or strictly control the
discharge of hazardous material such as heavy metals
into the environment. The importance of preventing or
precipitate as amorphous hydroxide [6], [7][8]. Because of its persistence in the environment, anthropogenic release of Cr6+ is a matter of [9].
Non-occupational exposure to the metal occurs
via the ingestion of chromium-containing food and water, whereas occupational exposure occurs via inhalation. Workers in the chromate industry have been exposed to estimated chromium levels of 10-50 µg/m3 for Cr (III) and 5-1000 µg/m3 for Cr (VI). Humans and animals localize chromium in the lung, liver, kidney, spleen, adrenals, plasma, bone marrow, and red blood cells (RBC). The main routes for the excretion of chromium are via the kidneys/urine and the bile/feces. Hexavalent chromium is transported into cells via the sulfate transport mechanisms, taking advantage of the similarity of sulfate and chromate with respect to their structure and charge. Once developed, chrome sensitivity can be persistent [9]. In such cases, contact with chromate-dyed textiles or wearing of chromate-
tanned leather shoes can cause or exacerbate contact
IJSEdermatitis. RVitamin C and other reducing agents
removing contamination of the environment with heavy
metals is paramount. It is widely known that such
metals contamination has dangerous effect on the flora
and fauna. The main sources of heavy-metal pollution are mining, milling and surface finishing industries, discharging a variety of toxic metals such as Cd, Cu, Ni, Co, Zn and Pb into the environment. In the last few decades, concentration of these heavy metals in river water/sediments has been clearly demonstrated [2]. Worldwide, chromium is known to be one of the most common inorganic contaminants of groundwater at pollutant hazardous sites. The anthropogenic inputs of chromium have increased rapidly since the industrial. Chromium is extensively used in electroplating (as chromeplating), resistant alloys (e.g., stainless steel), leather tanneries and dye productions. Chromium exists in a wide range of valency states from 4 to +6, with the hexavalent species (Cr6+) predominant in natural aquifers and its trivalent counterpart (Cr3+) prevailing in the municipal wastewater rich in organics. Hexavalent chromium poses a health risk to all forms of life [3], [4]. Considering its potential for hazardous toxicity and exposure, Cr (VI) has been designated as a priority pollutant in many countries [5]. Apart from its toxicity (discussed in Section 2), Cr6+ is also highly soluble and thus mobile and biologically available in the ecosystems. In contrast, Cr3+ displays a high affinity for organics resulting in the formation of complexes that
IJSER © 2013
combine with chromate to give Cr (III) products inside the cell. Hexavalent chromium compounds are genotoxic carcinogens. Chronic inhalation of hexavalent chromium compounds increases risk of lung cancer (lungs are especially vulnerable, followed by fine capillaries in kidneys and intestine) [5], [10].
Conventional techniques for metal removal from waste water includes chemical precipitation, ion exchange, membrane separation, evaporative distillation, liquid-liquid extraction, solvent extraction. These techniques are however becoming increasingly expensive and inefficient as stricter statutory limits for waste disposal are being introduced. Therefore there is an urgent need for the development of cost effective and efficient technology that could teat metal containing waste. Industries that produce waste water containing heavy water traditionally rely on physical-chemical processes to treat these waste. Metals bioremediation is still lagging behind in terms of commercial development. The present treatment technology involving physic-chemical and biological methods are not efficient and /or effective to treat the contaminants to acceptable level. Today, biotechnology is being considered as emerging science for environmental protection. The technology involves the use of microorganisms for biological treatment of air, water and soil pollutants. Biotechnological treatment is carried out at lower temperature and pressure which requires
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1842
ISSN 2229-5518
less energy than the conventional physico-chemical treatment technology. The industries generating
hazardous wastes have found beneficial measures from the emerging trend of biotechnological treatment. Biotechnological innovations for treatment for hazardous waste under controlled environmental conditions have been found cost–effective means of reducing the pollution potential of waste water, leading to enhanced public acceptance and compliance with environmental legislation [11].
Bioremediation of chromium extant in waste
involves different strategies that include biosorption,
bioaccumulation, bioreduction, and immobilization of biomaterial(s). Biosorption is a nondirected physiochemical interaction that occurs between metal species and the cellular components of biological species. It is metabolism-dependent when living biomass is employed, and metabolism-independent in dead cell biomass. Dead cell biomass is much more effective than living cell biomass at biosorping heavy
to either precipitate effectively immobilize inorganic pollutants such as heavy metals [12]. Stimulation of
microorganisms is achieved by the addition of growth substances, nutrients, terminal electron acceptor/donors or some combination thereby resulting in an increase in organic pollutant degradation and bio-transformation. The energy and carbon are obtained through the metabolism of organic compounds by the microbes involved in bioremediation processes [13].
Bioremediation technology uses micro- organisms to reduce, eliminate or transform contaminants present in soils, sediments or water. Bioremediation depends on the presence of specific microorganisms in the correct amounts and combination and in the appropriate environmental conditions [14]. Microorganisms living already living in contaminated environments are often well adapted to survive in the presence of existing contaminants and to the temperature, pH and oxidation/ reduction potential of the site. These indigenous microbes tend to utilize the
IJSER
metals, including chromium. Bioaccumulation is a
metabolically active process in living organisms that
works through adsorption, intracellular accumulation, and bioprecipitation mechanisms. In bioreduction processes, microorganisms alter the oxidation/reduction state of toxic metals through direct or indirect biological and chemical process(es).Bioreduction of Cr6+ to Cr3+ not only decreases the chromium toxicity to living organisms, but also helps precipitate chromium at a neutral pH for further physical removal ,thus offering promise as a bioremediation strategy. However, biosorption, bioaccumulation, and bioreduction methods that rely on free cells for bioremediation suffer from Cr6 toxicity, and cell damage. Therefore, immobilization of microbial cell biomass enhances bioremediation and renders industrial bioremediation processes more economically viable from reduced free- cells toxicity, easier separation of biosorbents from the tannery effluent, ability to achieve multiple biosorption cycles, and desorption (elution) of metal(s) from matrices for reuse. Thus, microbial bioremediation can be a cost competitive strategy and beneficial bioresource for removing many hazardous contaminants from tannery and other industrial wastes[3], [5].
Bioremediation is defined as the process by which microorganisms are stimulated to rapidly degrade hazardous organic pollutants to environmentally safe levels in soils, sediments, substances, materials and ground water. Recently, biological remediation process have also been devised
nutrients and electron acceptors that are available,
provided liquid water is present. Water also acts as a
vehicle to transport both microorganism and dissolved substances including contaminants and their breakdown products. Bioremediation process involves biotransformation and biodegradation by transforming contaminants to non–hazardous or less hazardous chemicals. Often, the microorganisms metabolize the chemicals to produce carbon dioxide or methane, water and biomass. Biotransformation is any alteration of the molecule or structure of a compound by micro- organisms. Biodegradation is the breaking down of organic or bioaccumulation and biotransformation of inorganic compounds into environmental friendly compounds.
Sewage and wastewater from different
industries contain heavy metals and conventional
treatment (activated sludge process) is not good enough to remove the metals rather the metals get accumulated in the sludge and subsequently enters the soil ecosystem through the soil and remaining metals in the treated wastewater and untreated wastewater increase the metal content of the water bodies and enters the ecosystem through aqueous ecosystem. Eventually, build-up of dangerous concentrations of toxic metals in grains and vegetables grown in contaminated soils is most alarming due to harmful effects of metals on human health. It is well known that heavy metals can be extremely toxic as they damage nerves, liver and bones, and also block functional groups of vital enzymes. Some
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1843
ISSN 2229-5518
of the metals like Ni are also listed as a possible human carcinogen (group 2B) and associated with reproductive
problems and birth defects [15].
Anaerobic digestion of wastewater and sludge
helps to reduce the pollution load but also produces H2 S. Presence of sulphide under reduced condition in the metal containing aqueous solution leads to metal- sulphide precipitation. Technology will be developed by utilizing this feature of the anaerobic treatment to remove metals from wastewater. Similarly, the leachate from landfill will be used as a typical wastewater which contains higher amount of metals. Anaerobic digestion of landfill leachate will be performed to remove organic load and metals [16]. Presence of H2 S in the biogas obtain from anaerobic digester reduces the usability of the biogas(methane) but metal-sulphide precipitation will help to reduce the H2 S content of the biogas and subsequently increase the value of biogas. Ultimately this method will not only remove the metals from wastewater, it will also generate cleaner biogas which
mg/m3 (for chromic acid & chromates and chromyl chloride listings)[5].
As human needs increase and civilization changes, more and more finished products of different types are required. Accordingly, large number of industries are born and grown in every country Process waste streams from the mining operations, metal- plating facilities, power generation facilities, electronic device manufacturing units, and tanneries may contain heavy metals at concentrations exceeding the local discharge limits. These waste streams contain toxic heavy metals such as chromium, cadmium, lead, mercury, nickel, and copper. They are not easily removed without specialized or advanced treatment. Chromium is a common pollutant introduced into natural waters / ground water due to the discharge of a variety of industrial wastewaters or chromium hazardous waste[14], [20],[21].
In India the Hazardous Waste Management
Law came in regulation in Year 1986 only, before that
IJSER
will have less hassle during combustion of biogas as less
H2 S content will reduce the probability of microbial
corrosion due to H2 S and also increase the calorific value of the biogas [17][18].
Presence of Cr (VI) more than the standard limit in the water bodies causes many adverse effects to human beings, animals, plants etc. Hence stringent regulations have been imposed by various organizations. According to the World Health Organization (WHO) drinking water guidelines, the maximum allowable limit for hexavalent chromium and total chromium (including Cr (III), Cr (VI) and other forms) are 0.05 and 2mg/L, respectively [19]. According to Safe Drinking Water Act, Maximum Contaminant Level (MCL) is 0.1 mg/L (total chromium). Maximum permissible level of chromium in bottled water is 0.1 mg/L. Specific color additives may contain chromium at levels no greater than 50 ppm. Chromium may be used in hydrolyzed leather meal used in feed for animals provided it contains chromium at levels below 2.75% of the total by weight. Occupational Safety and Health Administration (OSHA) prescribes the Permissible Exposure Limit (PEL) for Cr (VI) as 0.1 mg/m3 (based on chromic acid & chromates listing). National Institute for Occupational Safety and Health (NIOSH) indicates Immediately Dangerous to Life and Health (IDLH) limit as 15 mg/m3 (as Chromium (VI)) (For chromic acid & chromates listing). Recommended Exposure Limit (time-weighted-average workday) is restricted to 0.001
there was no regulation on such type of hazardous
waste disposal. The Present study deals with the
groundwater contamination due to industrial source in Lohiya Nagar industrial area of Ghaziabad, (U.P) India.Lohiya Nagar, situated in Ghaziabad, is spread over a vast area of several sq. m. land. Lohiya Nagar has got about 25% covered area and the remaining is open space. The Area under study is surrounded by Ghaziabad Town – which is comparatively high land and Hindon River in the Western Part – which is topographically a low land. Baba Bhim Nagar and Ambedkar Nagar come in the long Southern part and Sanjay Nagar, etc. come in its long Northern territory [22]. In India most of the contaminated sites are affected by hexavalent chromium from the illegal hazardous waste dumps containing Cr+6 such electroplating waste sludge and disposal of basic chrome sulphate waste. Uncontrolled & untreated discharge of electroplating waste such as Cr, Ni, Cu or CN waste on land or the sludges from the treatment plants having heavy metals or chemicals such as of basic chrome sulphate (used in leather tanning) can cause leachate and contaminate the ground water with Cr+6, Cu, Ni, etc. In Ghaziabad district there are numbers of Industries involving Cr & Ni electroplating which is a potential source of ground water contamination. Thus it is seen that the contamination of ground water in Lohiya Nagar is man- made because of uncontrolled discharge of electroplating waste in the past.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1844
ISSN 2229-5518
Groundwater quality studies were conducted by
collecting groundwater samples from the Lohiya Nagar
Ghaziabad area. The samples were analyzed in the laboratory following the IS-10500 norms. The
Groundwater Quality was tested as per IS:10500, the recommended code for Drinking Water Standard by the Indian Standards Bureau. The following Table provides an idea about the water quality in different locations S to S25 in the different areas of Lohiya Nagar of Ghaziabad
IJSER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1845
ISSN 2229-5518
12. | Phosphate | mg/L | - | 10 – 20 |
BIOLOGICAL PARAMETERS | ||||
1. | Total Coli form | MPN/100 ml | NIL | < 2.0 |
The depth of water level in the study area is shallowest in the close vicinity of Hindon River side and deeper water tables occurs towards Meerut Road side. The shallowest water table occurs at a depth of 22-26 m below the existing ground level. The deepest water table occurs at the depth of 26.5 m b.g.l (below ground level).
as an existing piezometer (PM), with well screens intersecting the same laterally- continuous Cr(VI)-impacted hydrostratigraphic unit consisting of sand lenses, inter-layered between clay and/or clayey caliche. The drilling program was carried out using water rotary drilling technique to advance each borehole to its termination depth of about 250 feet below
ground surface (bgs). Refer to the Well Field
IJSELocRation Plan for exploration locations relative
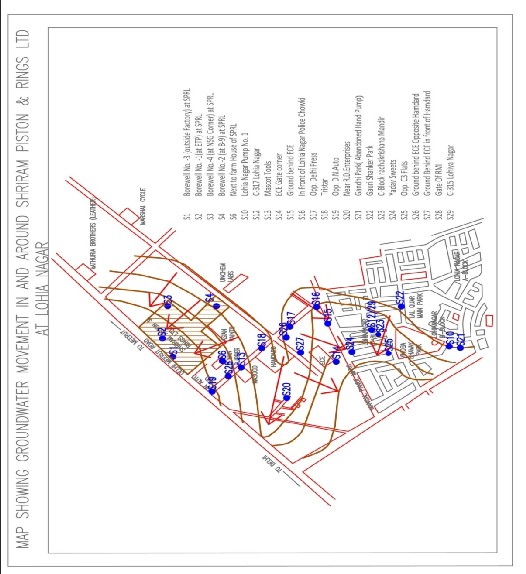
The groundwater flow in the study area is from upland,
i.e. from northwest side to south east side. The
groundwater potential is the capacity of the aquifer or groundwater reservoir to discharge water. This capacity or potential is low in areas lying in the southern and south-eastern part. High potential area occurs in the north-western part and in between the two lies the area with medium groundwater potential.
To evaluate an in-situ bioremediation approach for addressing the Cr(VI) contamination in overburden groundwater, the following work was completed:
1. Macro-level investigation of groundwater quality, in a quasi grid of 3 productive water supply wells screened in the shallow groundwater system, was carried out to assess the spatial distribution of Cr(VI) and other associated ions and also to locate highest Cr(IV) impacted area in which to carry out the remedial pilot study. Please refer to the Pilot Study Location Plan for the approximate location of the pilot study within the Study Area
2. Installation of a well field comprised of 21 new injection/monitoring wells (S1, S2…S21) as well
to the general direction of groundwater flow
(figure 1).
3. The baseline monitoring program was executed using a modified low-flow sampling technique. The purpose was to establish baseline groundwater quality conditions for the pilot study from which performance could be evaluated.
4. The remedial additive injection program, using a groundwater recirculation approach, was executed by achieving steady state pumping conditions, with a recirculation rate of about
300 liter per minute (LPM).
5. Remedial additive injection into the injection well and sampling well was carried out for about 20 hours at about 300 LPM mean flow rate (4 to 21 LPM range), for a total injection load of about 300 kilograms of EDC-M (Electron Donor Compound-Metals) dissolved in about 200 liters of re-circulated groundwater. Note that EDC-M is formulated with two components, EDC-M1 and EDC-M2, each of which is packaged separately and injected sequentially (i.e., EDC-M1 before EDC-M2).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1846
ISSN 2229-5518
6. Five rounds of (post-injection) performance monitoring were performed (same wells and parameters) using similar sampling technique, as for baseline sampling. Post-injection groundwater sampling results are also summarized in the Data Summary table 1.
The analysis further shows that the 02
Trough in the area has been recorded which are
as follows: First trough in between Dewan Rubber and Muscat and Second trough at Banke Bihari Temple. These troughs are converted into plumes of hexavalent chromium and the concentration level becomes high due to natural trough formation supported by low permeable and high porous strata. The plumes map is shown as below in figure 2.
The cross sections of these plume areas further indicated that at about 190 – 200 feet the concentrations of the hexavalent chromium high to medium. However, the concentration level of this plumed chromium reduces with depth. Evidently, in the pumping station of the Lohiya Nagar Pump House, the concentration of the hexavalent chromium is within permissible limits.
The lithological sections in terms of the vertical and lateral lithologies were analyzed using the fence diagram techniques and four types of the lithologies were encountered down to a depth of 300 feet. These lithologies of the soils are as follows: Top Soil as sandy silt with medium to low permeability and high porosity. Clay + Kankar formation with low to very low permeability and high porosity. Fine to Medium sand low to very low porosity and medium permeability.
IJSER
The study area is located in Lohiya Nagar of Ghaziabad
district. The area comprises of a number of the
industries of various nature including electroplating & various engineering units, etc. employing chromium electroplating as one of there processes. The same time the area has got residential pockets also. The impact of the groundwater contamination occurs in almost the entire part of the study area except few localized pockets. About 21 sounding stations and 20 water sampling stations have been chosen on the basis of hydrogeological spectrum of the area.
Coarse Sand very low porosity and high permeability.It
is seen in the area that the clay kankar are the main chromium accumulation regime and wherever the thickness of this is more the concentration of the hexavalent chromium is more. However, the topographical constraints are always exceptions. Reversely, the less thickness of the clay kankar reduces the Hexavalent chromium concentration.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1847

ISSN 2229-5518
IJSER
Figure 1: Map showing groundwater movement in and around shriram piston rings ltd. Dots show injection wells
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1848
ISSN 2229-5518
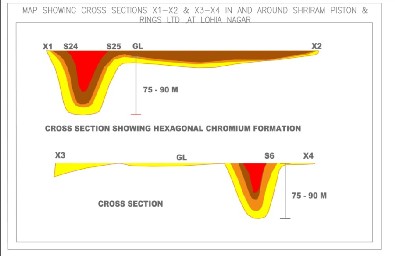
Figure 2 : MAP SHOWING HEXAVALENT CHROMIUM VALUES
LEGEND (Hexavalent Chromium in mg/l)![]()
12.0 – 16.0
8.0 – 12.0![]()
4.0 – 8.0![]()
1.0 – 4.0
IJSER
In Order to remove the groundwater contamination due to Hexavalent Chromium in certain areas of Lohiya Nagar, Ghaziabad and in order to stress the concept of Groundwater Remediation, an initiative by addressing the issue. Based on preliminary investigations, the pollution of groundwater due to Hexavalent chromium must be undertaken by Bioremediation Technique.
The Study is based on ‘Bio-Stimulation’ of Pseudomonas group of naturally occurring bacteria (Pseudomonas fluorescence, P. ambigua) in the sub-surface regime. The injection of EDC which is basically an electron donor, activates development of anaerobic conditions as a result of metabolism of organic carbon and ensuing bio-geochemical action [23]. The action of EDC chemically transforms Hexavalent chromium into trivalent form, which is insoluble in water and gets precipitated. The precipitated trivalent chromium gets further filtered by soil matrix [24]. The groundwater ultimately becomes free from chromium. This reduction
process is almost irreversible under natural and normal
groundwater conditions.
Three mechanisms by which microbes detoxify hexavalent Cr are :- Direct enzymatic (Cr6 reductase, Cytochrome–3 hydrogenase, flavin reductase) detoxification; Use of Cr (VI) as terminal electron acceptor in respiration process;Indirect reduction of Cr (VI) by the metabolic byproducts.Use of Bio-stimulant approach by stimulating native microflora supplying appropriate combination of energy source, vitamins, amino acids, trace elements and other proprietary growth promoting substances.
Some of the examples of microorganisms involved in the process: Pseudomonas sp., P. ambigua, Microbacterium, Aeromonas, Defulfomaculum, Arthrobacter, Bacillus sp. Shewanella oneidensis, Corynebacterium hoagie, Streptomyces thermocarboxydus, Pseudomonas fluorescence, Cellulomonas flavigena [25].
The injection process is executed under constant head conditions during which injection flow of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1849
ISSN 2229-5518
EDC slurry through the injection well and the static water level in all the remaining monitoring wells of the
well field are kept reasonably unchanged. In order to have proper impact of the EDC injection, one round of pre-injection monitoring and five rounds of post- injection monitoring must performed in total period of about three months. The parameters monitored included – Static Water level, pH, dissolved oxygen, oxidation- reduction potential (ORP), TOC, specific
conductivity, sulfate, nitrate, Cr(VI), Cr(total), Dissolved oxygen (DO), Oxidation reduction potential (ORP),
Sulfate, and Nitrate were identified as indicator parameters that control and are themselves affected by the biologically-mediated transformation of Chromium (VI) to Chromium (III)
12.0
10.0
8.0
6.0
4.0
2.0
0.0
ABSTRACTION WELL -
1
(Near Temple) ABSTRACTION WELL -
2
(Park Near C-292 ) ABSTRACTION WELL -
3
(Green Belt)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
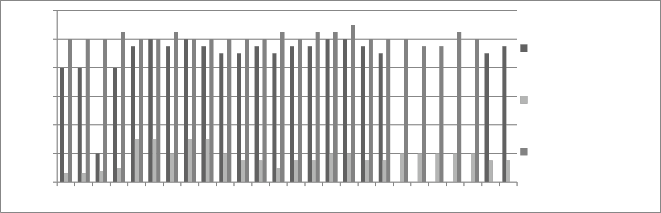
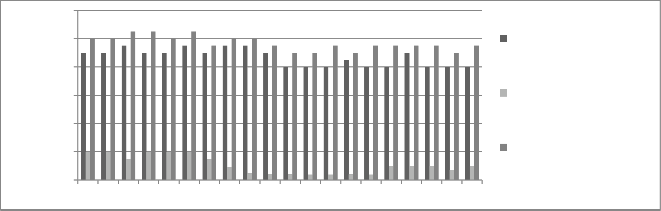
Figure 3: Chromium concentration in month of June 2012
12.0
10.0
8.0
6.0
4.0
2.0
0.0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
ABSTRACTION WELL -1 (Near Temple)
ABSTRACTION WELL -2 (Park Near C-292 )
ABSTRACTION WELL -3 (Green Belt)
Figure 4: Chromium concentration in month of July 2012
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1850
ISSN 2229-5518
12.0
10.0
8.0
6.0
4.0
ABSTRACTION WELL -1
(Near Temple)
ABSTRACTION WELL -2
(Park Near C-292 )
2.0
0.0
1 2 3 4 6 7 8 9 11 12 13 14 16 17 18 20 21 22 23 24 25 27 28 29 30 31
ABSTRACTION WELL -3 (Green Belt)
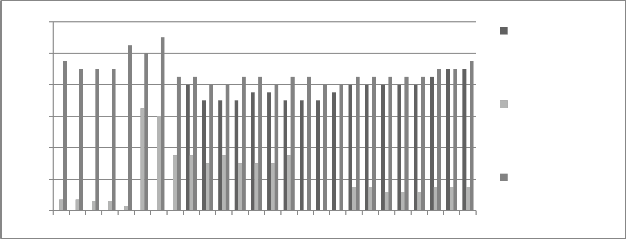
Figure 5: Chromium concentration in month of August 2012
14.0
12.0
10.0
8.0
6.0
4.0
2.0
0.0
ABSTRACTION WELL -
1
(Near Temple) ABSTRACTION WELL -
2
(Park Near C-292 ) ABSTRACTION WELL -
3
(Green Belt)
1 3 4 5 6 7 8 10 11 12 13 14 15 17 18 19 20 21 22 24 25 26 27
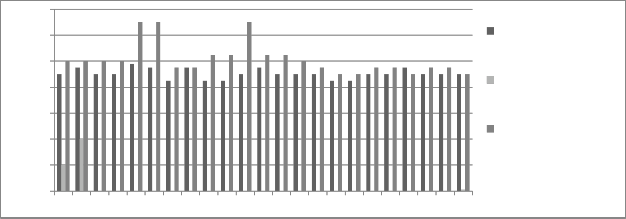
Figure 6: Chromium concentration in month of September 2012
12.0
10.0
8.0
6.0
4.0
2.0
0.0
1 3 4 5 6 8 9 10 11 12 13 15 16 17 18 19 20 22 23 24 25 26 27 29 30
ABSTRACTION WELL -1
(Near Temple)
ABSTRACTION WELL -2
(Park Near C-292 )
ABSTRACTION WELL -3 (Green Belt)
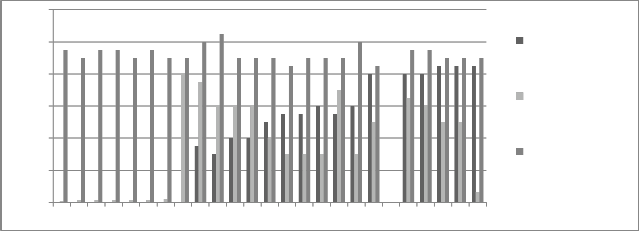
Figure 7: Chromium concentration in month of October 2012
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1851
ISSN 2229-5518
12.0
10.0
8.0
6.0
4.0
2.0
0.0
1 2 3 5 6 7 8 9 10 12 13 14 15 16 17 19 20 21 22 23 24 26 27 28 29 30
ABSTRACTION WELL -1
(Near Temple) ABSTRACTION
WELL -2
(Park Near C-292 ) ABSTRACTION
WELL -3
(Green Belt)
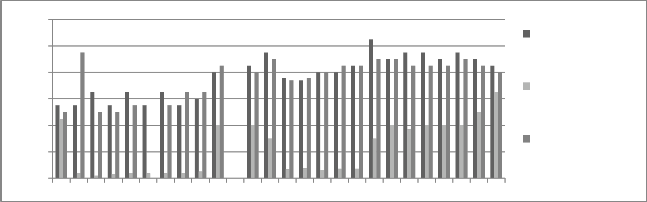
Figure 8: Chromium concentration in month of November 2012
10.0
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
IJSER
1 2 3 4 5 7 8 9 10 11 12 14 15 16 17 18 19 21 22 23
ABSTRACTION WELL -1
(Near Temple)
ABSTRACTION WELL -2
(Park Near C-
292 )
ABSTRACTION WELL -3 (Green Belt)
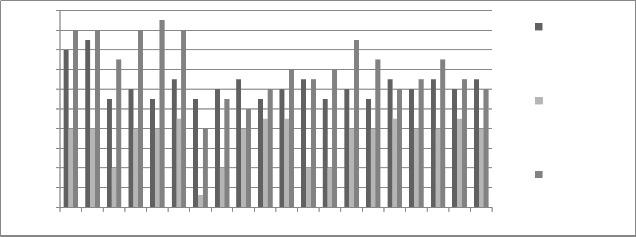
Figure 9: Chromium concentration in month of January 2013
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
1 2 3 4 5 6 7 8 9 11 12 13 14 15
ABSTRACTION WELL -1
(Near Temple)
ABSTRACTION WELL -2
(Park Near C-292
) ABSTRACTION
WELL -3 (Green Belt)
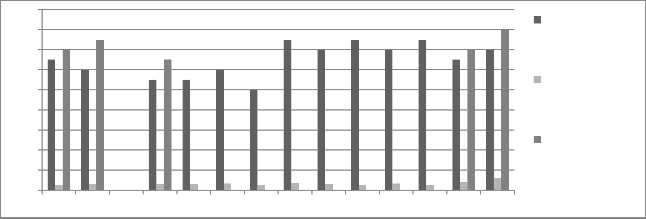
Figure 10: Chromium concentration in month of February 2013
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1852
ISSN 2229-5518
12.0
10.0
8.0
6.0
4.0
2.0
0.0
1 2 4 5 6 7 8 9
ABSTRACTION WELL
-1
(Near Temple) ABSTRACTION WELL
-2
(Park Near C-292 ) ABSTRACTION WELL
-3
(Green Belt)
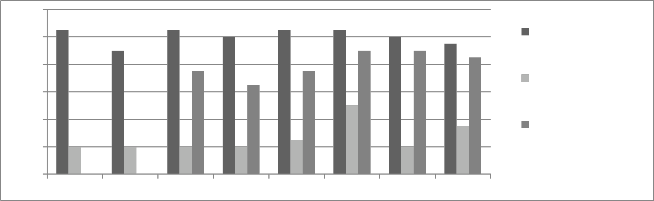
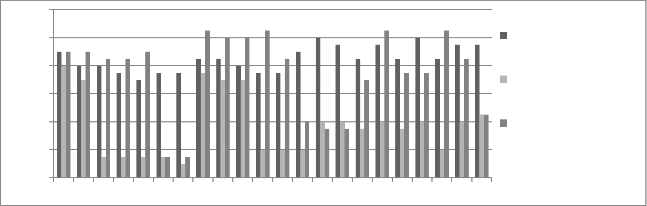
Figure 11: Chromium concentration in month of March 2013
12.0
10.0
8.0
6.0
4.0
2.0
ABSTRACTION WELL -1 (Near Temple)
ABSTRACTION WELL -2 (Park Near C-292 )
ABSTRACTION WELL -3 (Green Belt)
0.0
1 2 3 4 5 6 8 9 10 11 12 13 15 16 17 18 19 20 22 23 24 25
Figure 12: Chromium concentration in month of April 2013
14.0
12.0
10.0
8.0
6.0
4.0
2.0
0.0
1 2 3 4 6 7 8 9 10 11 13 14 15 16 17 18 20 21 22 23 24 25 27 28 29 30 31
ABSTRACTION WELL -1 (Near Temple)
ABSTRACTION WELL -2 (Park Near C-292 )
ABSTRACTION WELL -3 (Green Belt)
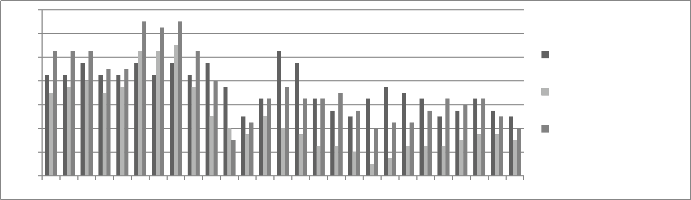
Figure 13: Chromium concentration in month of May 2013
Graphs are plotted against days of month with respect
to chromium concentration. The initial chromium concentration in the ground water was 12.4 mg/l, 11.4 mg/l and 12.4 mg/l in abstraction well 1, 2 and 3
respectively. The monthly monitoring results are
present in the figures (5-15). Abstraction well 2 has shown tremendous decrease in the initial concentration. The results show that for initially few months the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1853
ISSN 2229-5518
concentration of chromium in the ground water decreased but increased in the month of August,
September and October (figure 5, 6 and 7). In the month of June and July (figure 3 and 4) the Cr concentration was between 8-10 mg/l which increased to 10-12 mg/l in the month of August, September and October (figure 5,6 and 7). The concentration again decreased after October i.e in the month of November to May (figure 8-13). The concentration was between 5-8 mg/l respectively. The reason in the considerably decrease in the concentration of abstraction well 2 may be because of the two tube well present near this abstraction well. The tube wells are in working conditions. These tube wells act as a pump and treat method. Which result in the decrease in the chromium concentration in ground water.
Increase in the Cr concentration in the month
of August, September and October is due to the rain
water which percolates down the ground and leach the Cr present above the ground water to water level resulting in the increase in concentration in ground
water. An increase in the chromium concentration was
positive results of the Remedial Pilot Study, it is suggested that the pump and treat method help in
reducing the heavy metal concentration by diluting the ground water.
This study is a part of a research project which is supported by SPRIL Authors are grateful to the managing Director of SPRIL Ghaziabad. Authors are also thankful to the Head and Dean Jamia Milia Islamia University, Delhi
[1] M. Mahmoud Abdel daiem, Jose Rivera-Utrilla, Raul Ocampo-Perez, Jose D. Mendez-Diaz and Manuel Sanchez-Polo, “Environmental Impact of Phthalic Acid Esters and Their Removal
From Water and Sediments by Different
IJSER
observed in the month of February to May (figure 10-
13). The water level during this season acquire the declining tread due to which there is a fall in the water level as a result the flushing of soil starts and the dilution of hexavalent chromium from to the water mixes as result the concentration increases due to dilution effect.
As inferred from the variation in the
concentration of indicator parameters over a period of the Pilot Study, the decrease in concentration of oxides i.e. sulphate, nitrate, sulphate and development of (-) potential of ORP is indicative of development of oxygen deficient groundwater regime as impacted by the remedial additive and associated stimulation of the soil bacteria. The oxygen deficient condition played key role in chemical transformation of chromium Hexavalent to its trivalent state. The latter being water insoluble remained in the soil matrix and resultant remediated groundwater eventually became free from corresponding reduction in chromium.
Considering successful execution of the Pilot study, it is proposed to undertake ‘Full Scale Remediation’ of the problem in the entire affected area spanning 4 square km. The prime cost component of EDC is liable to be cut down significantly. In this way the treated groundwater will be used for Agriculture in the surrounding areas and for construction activity and also for Cooling Tower, Air Conditions and Comfort Cooling in the entire Industrial area. Based on the
IJSER © 2013 http://www.ijser.org
Technologies - A Review,” J. Environmental
Management, vol.109, pp 164-78, Oct 2012.
[2] V.L Colina, L.B Villegasa and C.M Abatea, “Indigenous Microorganisms as Potential Bioremediators for Environments Contaminated with Heavy Metals,” International Biodeterioration & Biodegradation, vol.69, pp28–37, 2012.
[3] SK Garg, M. Tripathi and T. Srinath,“Strategies
for Chromium Bioremediation of Tannery
Effluent, Review in Environmental
Contamination and Toxicology, vol 217, pp75-
140, 2012.
[4] M. Mohanty and H.K Patra, “Attenuation of
Chromium Toxicity by Bioremediation
Technology. Review in Environmental
Contamination and Toxicology, vol. 210 pp 1-
34, 2011
[5] A.P. Das, “Bioreduction based bioremediation
of hexavalent chromium Cr (VI) through
potential indigenous microbes,” Ph.D dissertation, Dept. of Chemical Eng., National Institute of Technology, Rourkela, 2009.
[6] C.D Palmer and P.R Wittbrodt, “ Processes Affecting the Remediation of Chromium- Contaminated Sites,” Environmental Health Perspectives vol. 92, pp 25-40, 1991.
[7] K. Parmar and S. Priya, “Evaluation of Ground
Water Quality of Jamshedpur City in
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1854
ISSN 2229-5518
Jharkhand,” International Journal of
Innovations in Bio-Sciences, vol. 3 no. 3, pp. 81-
86, 2013.
[8] P. Vajpayee, U. N. Rai, M. B. Ali, R. D. Tripathi,
V. Yadav, S. Sinha, S. N. Singh, Chromium- Induced Physiologic Changes in Vallisneriaspiralis L. and Its Role in Phytoremediation of Tannery Effluent, Bull. Environ. Contam. Toxicol.vol. 67 pp246–256.
2001.
[9] K.H. Cheunga and Ji-Dong Gu, “Mechanism of
Hexavalent Chromium Detoxification by
Microorganisms and Bioremediation Application Potential: A Review, International Biodeterioration & Biodegradation, vol. 59, pp
8–15, 2007.
[10] V. Hassmanova ,J. Vaneckova and K. Bousova,
Occupational Diseases Caused by Chromium and its Compounds,” Acta Medica (Hradec Kralove) Suppl. vol.43, no. 1, pp 33-6, 2000.
[18] B. Pandey, M.H Fulekar, “Bioremediation
Technology: A New Horizon for Environmental
Clean-Up, vol. 4 no. 1, 51-59, 2012.
[19] V.K Gupta and A. Rastogi, "Biosorption of
Hexavalent Chromium by Raw and Acidtreated Green Alga Oedogonium Hatei From Aqueous Solutions, J. Hazardous Materials ,vol. 163,no. 1, pp. 396-402, 2009.
[20] M. Vidali, Bioremediation. An overview, Pure
Appl. Chem., vol. 73, No. 7, pp. 1163–1172, 2001 [21] Derek R Lovley and John D Coates, “Bioremediation of metal Contamination,” Current Opinion in Biotechnology,” vol.8,
no.3, pp. 285-289,1997.
[22] www.cgwb.gov.in
[23] C. R Evanko, and D.A Dzombak,. Remediation of Metals-Contaminated Soil and Groundwater. Ground-Water Remediation Technologies Analysis Center (GWRTAC) Office of Solid Waste and Emergency
IJSER
[11] M.H Fulekar, “Bioremediation Technology,”
Recent Advances, pp 135-166, 2010.
[12] M.C Vargas-Garcia, M. J Lopez, F. Suarez- Estrella and J. Moreno, “Compost as a Source of Microbial Isolates for the Bioremediation of Heavy Metals: In Vitro Selection, Science of the Total Environment,” vol. 431, no. 1,62-67, 2012.
[13] M.H Fulekar, “Bioremediation of Fenvalerate by Pseudomonas Aeruginosa in a Scale up Bioreactor,” Romanian Biotechnological Letters, vol. 14 no.6, 4900-4905, 2009.
[14] R. Boopathy, Factors Limiting Bioremediation
Technologies,” Bioresource Technology, vol.74 pp. 63-67, 2000.
[15] A. Malik., “Metal Bioremediation Through
Growing Cells,” Environment International, vol
30, pp 261 – 278, 2004.
[16] Mohammad Saghir Khan, Almas Zaidi, Reeta Goel, Javed Musarrat, “Biomanagement of Metal- Contaminated Soils,” Environmental Pollution, vol. 20, 2011.
[17] Robert Paul van Hille, Biological Generation of Reactive Alkaline Species and Their Application in a Sustainable Bioprocess for the Remediation of Acid And Metal Contaminated Wastewaters,” Department of Biochemistry and Microbiology, Rhodes University, Dec
2001.
Response Technology Innovation. U.S.
Environmental Protection Agency
Washington, DC, pp. 61. 1997
[24] Ala Eldin M.E.H. Sami Ahmad M., Gurunadha
Rao, Dhar R.L. (1998): Groundwater Modelling
In Parts of Central Ganga Basin, Uttar Pradesh, India. Technical Report. No. AMU/NGRI-1, Collaborative Project between Remote Sensing Application for Evaluation and Geo- Engineering, Aligarh and NGRI.
[25] Abhipsa Sarangi and Chandraraj Krishnan, Indian Institute of Technology, Chennai, ENVIS CENTRE Newsletter vol..7 ,no.2, 2009. Available at http://www.envismadrasuniv.org/nl20092%20a rticles%20Enzymatic%20reduction.html.
IJSER © 2013 http://www.ijser.org