International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 995
ISSN 2229-5518
Hormonal effect on shoot multiplication in
Sugarcane genotypes
Syed Rizwan Abbas1, Syed Dilnawaz Ahmad Gardazi1, Wajid Aziz3, Sardar Ali Khan1, Syed Mubashir Sabir2, Attiya Batool4, Muhammad Rehan Abbas3 and Sundas Shahzad5.
1Department of Plant Breeding and Molecular Genetics, Faculty of Agriculture, University of Azad Jammu and
Kashmir, Muzaffarabad A.K. Pakistan
2Faculty of Agriculture, University of Poonch, Rawalakot, A.J.K. Pakistan
3Department of Computer Science, University of Azad Jammu and Kashmir, Muzaffarabad A.K. Pakistan
4Department of Botany, University of Azad Jammu and Kashmir, Muzaffarabad A.K. Pakistan
5Department of Biotechnology, Ayub Agriculture Research Institute, Faisalabad, Pakistan
*Address for Author: Syed Rizwan Abbas, Department of Plant Breeding and Molecular Genetics, Faculty of
Agriculture, University of Azad Jammu and Kashmir, Muzaffarabad, Pakistan
E-mail: drsyedrizwanabbas@gmail.com
Studies were carried out for rapid micro propagation of five sugarcane genotypes i.e.HSF-242, SPF-213 HSF-240, CP-77-400 and CP-43-33. The explants were surface sterilized with 40% clorex for 45 minutes. Multiplication of the cultures was obtained by using BAP and Kinitin in various combinations and concentrations in MS medium. The optimum multiplication for genotype HSF-240 was obtained at 1.5 mg/l BAP, 0.5 mg/l Kin with 12.6 cm shoot, 11tillers and 8 leaves per plant. Similarly optimum multiplication for variety CP-77-400 was obtained at 0.5 mg/l BAP and 1.0 mg/l Kinitin, with a maximum of 9 cm shoot length, 1 tiller and 16 leaves. The variety SPF-213 was obtained at 1.5 mg/l BAP and 0.1 mg/l Kin, with a maximum of 9.6 cm shoot length, 8 tillers and 11leaves. Best multiplication rate for variety HSF-242 and CP-43-33 was observed at 1.5 mg/l BAP and 0.1 mg/l Kin with a maximum of 13 cm shoot, 8 tillers and 10 leaves per plant and 1.0 mg/l BAP and 0.1 mg/l Kin with a maximum of 10 cm shoot, 11 tillers and 12 leaves per plant. Rooting of the plantlets was obtained on half strength MS medium containing 6% sucrose and various concentrations of IBA. The experiment will be help for further selection and multiplication of desirable sugarcane varieties.
Keywords: Sugarcane, kinetin, Multiplication, BAP, IBA
Sugarcane crop is a major raw material source for the production of white sugar, brown sugar and gur and is also a cash crop. Its share in value addition in agriculture and GDP is 3.6 and 0.8 percent, respectively. Sugarcane was cultivated on an area of 988 thousand hectares during the year 2010-11, 4.8 percent higher than last year’s level of 943 thousand hectares production for the year 2010-11 is estimated at 55.3 million tons as against actual production of 49.3 million for the year 2009-10 (MINFA, 2011). Commercially, sugarcane is propagated from stem cuttings with each cutting or set having two or three buds. After the establishment of variety, major bottleneck in spreading of the variety is slow propagation rate through conventional method, which takes years (Cheema & Hussain, 2004). Modern commercial sugarcane varieties are obtained through breeding and a multi-stage selection scheme over a period of 10-15 years. Tissue culture techniques have been widely used for large-scale micro propagation and can effectively reduces the time period between selection and commercial release of new sugarcane varieties (Lorenzo et al., 2001; Taylor, 1997). Micro propagation is currently the only realistic means of achieving rapid, large-scale production of disease-free seed canes of newly
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 996
ISSN 2229-5518
developed varieties in order to speed up the breeding and commercialization process in sugarcane (Feldmann et al., 1994; Lal & Krishna, 1994). In contrast to conventional method where one bud produces 4-5 shoots, tissue culture, if estimated conservatively can produce around 10,000 identical plants from a single bud in about 3-4 months (Lee, 1987). Micropropagation can also be used for obtaining disease free plants. Many scientists have described methods for micropropagation (Hendre et al., 1983, Gosal et al., 1998, Jadhav et al., 2001). Gosal et al., (1998) reported rapid multiplication in liquid MS medium on BAP (0.5 mg/l) and Kin (0.5 mg/l) and rooting on NAA (0.5 mg/l) and sucrose 70%. In the present study we was to developed protocol for fast and efficient micro propagation of five sugarcane genotypes viz., HSF-242, SPF-213, HSF-240, CP-77-400 and CP-43-33 by using BAP and Kinetin in various concentrations and combinations, which will help to cope with the increasing demand for sugarcane production on large scale to meet the future challenges for sugar production.
The plant materials were collected form glasshouse of faculty of agriculture Rawalakot and all the experimental work was carried out at Plant tissue culture lab Department of Plant Breeding and Molecular Genetics. Five sugarcane varieties HSF-242, HSF-240, CP-77-400, SPF-213 and CP-43-33 were used in this study. The explants materials were taken from six months old sugarcane plants.
Size of the shoot tip taken was 4-6 mm. For surface sterilization of explants 40% Clorox (commercial bleach containing 5.25% v/v Sodium hypochlorite) for 45 minutes was used but before clorex treatment the explants were first thoroughly washed with tap water for several times, then put in 60% ethanol for 30 minutes and finally in antioxidants solution for 45 minutes. The explants were washed with autoclaved distilled water thrice time for 5 minutes.
Solid MS (Murashige & Skoog`s medium, 1962) supplemented with 0.1 mg/l GA3 and 1.0 mg/l Kin for initiation of cultures, various concentrations and combinations of BAP and Kin in liquid MS medium for the multiplication of cultures and with 0.5 mg/l-1.5 mg/l of IBA with 6% sucrose for the rooting of cultures. Data for shoot initiation were recorded 30 days after culturing, for shoot multiplication 30 days and 15 days for rooting after culturing. Data were analyzed using a computer package MSTAT.
Multiplication of shoots was observed at different concentrations of BAP in combination with Kin. The varieties indicated variable towards the different concentrations used, however BAP (0.5, 1.5-1.0 mg/l) with Kin (1.0, 0.1-0.5 mg/l) were found best for multiple shoots formation. At maximum of 13 cm long shoot produced 11 tillers and 16 leaves per plant on different combinations in 30 days.
The average shoot length found from varieties HSF-242, HSF-240, SPF-213, CP-43-33 and CP-77-40 were highly
significantly different. The shoot length ranged from 9 cm for variety CP-77-400 to 12.6 cm for variety HSF-242
(Fig-3). The impact of different hormones combinations on average shoot length was also highly significantly
different.
The impact of tillers between genotypes showed less difference. However the number of tillers ranged from 8
in variety HSF-240 and SPF-213 to 12 in CP-77-400 (Fig-2). The different hormones combinations showed
highly significant impact on average number of tillers. The highest value of tillers was observed at T3 (BAP-1.5,
Kin.-0.5) while the lowest one was for that of control T2 (Fig: 2). Rapid shoot multiplication was achieved in
liquid MS medium supplemented with cytokinins, Kin (0.5 mg/l) and BAP (0.5 mg/l) with 20 shoots per plant (Gosal et al., 1998). The interaction effect between varieties and different hormones combinations was non- significantly different on tillers number (Fig-2). The average number of leaves for different varieties (HSF-242, HSF-240, CP-77-400,CP-43-33 and SPF-213) were highly significantly different. The means for number of leaves
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 997
ISSN 2229-5518
ranged from 10 for variety HSF-242 to 16 for that of variety CP-77-400 (Fig: 1). Chattha et al., (2001) reported that multiple shoots formation was excellent at 1.5 mg/l BAP in combination with 1.5 mg/l GA3 and recorded a maximum of 4 tillers and 16 leaves per plant. The impact of different hormones combinations was also highly significant against leaves number. However the leaves number ranged from 10 as the lowest value for T2 to 16 as the highest value for T4 (Fig: 1). Patel et al., (2001) recorded highest multiplication on 1.5 mg/l Kin. The combinations T1, T3 and T4 were highly significantly different while the combinations T2, T3 and T5 were non- significantly different (Fig: 1).
![]() T1
T1 ![]() T2
T2 ![]() T3
T3 ![]() T4
T4 ![]() T5
T5
20
10 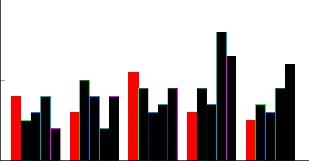
0
HSF- 240 HSF- 242 SPF- 213 CP- 77- 400 CP- 43- 33
Fi g: 1 com par esi on bet w een no of l eaves at f i ve hor m onal t r eat m ent
![]() T1
T1 ![]() T2
T2 ![]() T3
T3 ![]() T4
T4 ![]() T5
T5
20
10 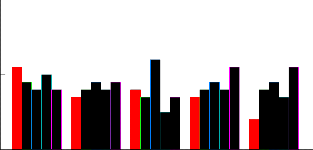
0
HSF- 240 HSF- 242 SPF- 213 CP- 77- 400 CP- 43- 33
Fi g: 2 com par esi on bet w een no of t i l l er s at f i ve hor m onal t r eat m ent s
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 998
ISSN 2229-5518
![]() T1
T1 ![]() T2
T2 ![]() T3
T3 ![]() T4
T4 ![]() T5
T5
20
10 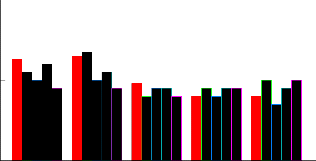
0
HSF- 240 HSF- 242 SPF- 213 CP- 77- 400 CP- 43- 33
Fi g: 3 com par esi on bet w een shoot l enght at f i ve hor m onal concent r at i ons
![]() T1
T1 ![]() T2
T2 ![]() T3
T3 ![]() T4
T4 ![]() T5
T5
10
9
8
7
6
5
4
3
2
1
0
HSF- 240 HSF- 242 SPF- 213 CP- 77- 400 CP- 43- 33
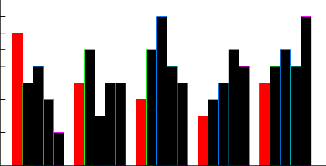
Fi g: 4 com par esi on bet w een f i ve genot ypes at f i ve hor m onal concent r at i ons
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 999
ISSN 2229-5518

HSF-242

HSF-240

IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1000
ISSN 2229-5518
CP-77-400

SPF-213

CP-43-33
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1001
ISSN 2229-5518
1. Baksha, R., R.Alam, M.Z.Karim, B.S.K.Paul, M.A.Hossain M.A.S.Miah and A.B.M.M.Rahman. 2002. In vitro shoot tip culture of sugarcane (Saccharum oficinarum) variety LSD28. Biotechnology, 1(2-4); 67-72.
2. Chattha, M.A., A.Abida, I.Muhammad and A.Akhtar.2001. Micropropagation of sugarcane (Saccharum species hybrid). Pak.
Sug. J., 16: 2-6.
3. Cheema, K.L. and M.Hussain.2004. Micropropagation of sugarcane through apical bud and axillary bud. Inter. J. of Agri. and
Biol., 2: 257-259.
4. Ministry of Food and Agriculture of Pakistan. 2011. Federal Bureau of Statistics.,18-19.
5. Feldmann, P., J.Sapotille, P.Gredoire and P.Rott. 1994. Micropropagation of sugarcane. In: In vitro culture of tropical plants. (Ed.): C. Teisson. France: CIRAD: 15-17.
6. Gallo-Meagher, M., R.G.English and A.Abouzid.2000. Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. In vitro Cell Dev. Biol. Plant, 36:37-40.
7. Geetha, S., D.Padmanabhan, W.W.Manuel and A.Ayyamperumal. 2000. In vitro production of sugarcane plants. Sugar Tech., 2:
3, 47-48.
8. Gosal, S.S., K.L.Thind and H.S.Dhaliwal.1998. Micropropagation of sugarcane. An efficient protocol for commercial plant
production. Crop Improv., 2: 167-171.
9. Hendre, R.R., R.S.Iyer M.Kotwal, S.S. Khuspe and A.F. Mascarenhas.1983. Rapid multiplication of sugarcane by tissue culture.
Sugarcane 1: 5-8.
10. Jadhav, A.B., E.R.Vaidya, V.B.Aher and A.M. Pawar. 2001. In vitro multiplication of co–86032 sugarcane (S. oficinarum) hybrid.
Indian J. Agric. Sci., 71: 113-115.
11. Lal, N. and R.Krishna. 1994. Sugarcane and its problems: Tissue culture for pure and disease free seed production in sugarcane. Indian sugar, 44: 847-848.
12. Lee, T.S.G. 1987. Micropropagation of sugarcane (Saccharum spp.) Plant Cell Tissue Org. Cult., 10: 47-55.
13. Lorezo, J.C., E.Ojeda, A.Espinosa and C.Borroto. 2001. Field performance of temporary immersion bioreactor derived
sugarcane plantys. In vitro cell Dev. Biol. Plant, 37: 803-806.
14. Mamun, M.A., M.B.H. Skidar, D.K. Paul, M.M. Rehman and M.Islam. 2004. In vitro micropropagation of some important
sugarcane varieties of Bangladesh. Asian J. of Plant Sci., 3(6): 666-669.
15. Mstat, C. 1991. Michigan State University, East Lansing, USA.
16. Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Pl. Physiol., 9:
473-497.
17. Patel, A.A., S.R. Patel, C.L.Patel and B.S.Prajapati. 2001. Effects of media composition on in vitro multiplication of sugarcane
varieties. Ind. J. Gene. Plant Breed., 61(1): 82-83.
18. Razi-ud-Din, S.S. Shah, S.W. Hussan, S.Ali and R.Zamir. 2004. Micro-propagation of sugarcane though bud culture. Sarhad J.
Agri. 20: 1.
19. Taylor, P.W.J. 1997. Micropropagation of sugarcane (Saccharum spp. Hybrid). In: Biotechnology in Agriculture and forestry, volume 39, high-tech and Micropropagation v. (Ed.): Y.P.S. Bajaj. Springer-verlag Berlin, 256-271.
20. Wongkaew, P., and J.Fletcher. 2004. Sugarcane white leaf phytoplasma in tissue culture, long term maintenance, transmission and oxytetracycline remission. Plant Cell Rep., 23: 426-434.
IJSER © 2013 http://www.ijser.org