International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1593
ISSN 2229-5518
High purity Alpha Alumina nanoparticle: Synthesis and characterization
Hamed Sadabadi1, Adeleh Aftabtalab2, Shirzad Zafarian1, Sarah Shaker1, Mohsen Ahmadipour1 and K.Venkateswara
Rao1
1. Center of Nano Science and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological university of Hyderabad, AP, India
2. Center for Environment, Institute of Science and Technology, Jawaharlal Nehru Technological university of Hyderabad, AP, India
Abstract - High purity crystalline alpha alumina (-Al2O3), platelet powder, synthesized by the combustion synthesis, aluminum nitrate was used as the source of aluminum and urea as oxidizer in an aqueous medium. X-Ray diffractometer applied to study crystalline phase. Scanning Electron Microscopy (SEM) and Energy dispersive X-ray spectroscopy analysis (EDAX) was used for morphological and chemical characterization of nanoparticel. Further study on crystalline structure of -Al2O3was done by applying Transmission Electron Microscopy (TEM) and Selected Area Electron Diffraction (SAED). Size distribution of powder investigated using Particle Size Analyzer (PSA). Thermal gravimetric and differential thermal analysis(TG/DTA)evaluated thermal behavior of -Al2O3.
Index Terms: -Al2O3, combustion synthesis, High purity, Nanostructure, platelet structure, SEM, TEM.
—————————— ——————————
1 INTRODUCTION
Alpha alumina, known as corundum, has been attraction attention as one of most important ceramic material due to its significant properties such as: high strength at elevated temperature, hardness, high melting point, thermal conductivity, chemical inertness, abrasion resistance and so on [1-3]. Such excellent properties made -Al2O3 nanoparticle promising material for a wide range of applications such as: electronics [4], optoelectronics [5], and reinforcement filler in composites [6,7]. Recently, -Al2O3 nanostructures such as: nanotube [8], nanowire, nanobelt [9], nanoplatelet [10] and spherical nanoparticle [11] have been received attraction in mechanical applications. Different methods used to synthesis alpha alumina nanostructures including: Chemical Vapor Deposition (CVD) [9], Combustion Chemical Deposition (CCD) [11], Atomic Layer Deposition (ALD) [12], and Spray pyrolysis [13], and. All these techniques require specific equipments, while industrial processes must be affordable, time- consuming and abundant production rate [14]. Solution combustion synthesis (SCS) is time efficient, low-cost, safe and environmental friendly procedure for metal oxide synthesis [15]. Furthermore, in techniques mentioned above approaching alpha phase, High-temperature annealing above 1100o C of production is mandatory [16-19]. In present work, -Al2O3 platelet nanostructure with high purity obtained directly from solution combustion procedure.
2 EXPERIMENTAL PROCEDURE
2.1. Materials:
Aluminum nitrate nonahydrate extra pure provided from E. Merck (India) limited Co. Urea extra pure purchased from Thomas Baker (chemicals) PVT limited Co (India).
2.2. Sample preparation
Crystalline -Al2O3 platelet nanostructure was synthesized by solution combustion. Mixture solution of aluminum nitrate and urea prepared by dissolving 15.0 gm aluminum nitrate in 20 ml distilled water using magnetic stirrer, 6.0 gm urea slowly added to the solution through vigorous stirring. Transparent solution placed on a pre- heated hot plate. Oxidizer to fuel ratio (factor) kept unit in this experiment. Obtained alpha alumina powder dehydrated at 450o C in an air furnace to improve purity.
3 RESULTS AND DISCUSION
D8 Advance (Bruker) X-ray diffractometer used to record XRD patterns, with Cu Kα irradiation by wavelength 1.54Å (40 kV, 40 mA). Morphological study and chemical characterization of powder were observed by Scanning Electron Microscopy (SEM) using a S-3400N (Hitachi High-Technologies, Japan) microscope,
Transmission electron microscopy (TEM), High resolution
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1594
ISSN 2229-5518
TEM (HR-TEM), and selected area electron diffraction (SAED) patterns obtained using a JEM-2100 (JEOL, Tokyo, Japan) microscope. The SZ-100 nanoparticla series instruments (Horiba, Kyoto, Japan) used to study particle size and thermal gravimetric analysis and differential thermal analysis (TG/DTA) performed in air at heating rate of 30o C/min, using a thermal analyzer model XSTAR6000 (Hitachi High-Tech Science Corporation, Tokyo).

Fig.1. XRD pattern of -Al2O3 synthesis by solution combustion method of aluminum nitrate and Urea in
20 ml distilled water.

Fig.2. SEM images of -Al2O3 sample, indicates platelet morphology.
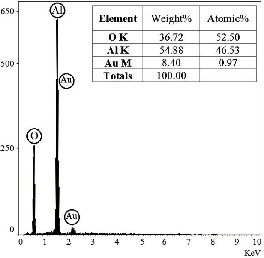
Fig.3. EDAX spectrum of -Al2O3 synthesized by solution combustion method.
Scanning Electron Microscopy (SEM) with Simultaneous EDAX was employed to study platelet morphology of product (Fig.2.) and EDAX spectrum confirms high purity of -Al2O3 (Fig.3.) and reveals that particle is composed of Al and O elements. Au element observed is due to preparation process for SEM, because alumina is ceramic material.
Fig.4. and Fig.5. show the TEM micrographs of -Al2O3
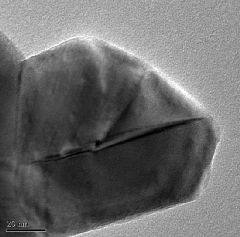
powder obtained from the solution combustion of Aluminum nitrate (oxidizer) and Urea (fuel) in 20 ml aqueous medium and desiccated at 450oC for 1 h. This shows that most crystallites exhibit hexagonal platelet morphology. The side lengths of the platelets were ~80 nm for 20 ml aqueous.
Fig.4. TEM micrograph of -Al2O3 nanoparticles shows hexagonal shape synthesized by SCS.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1595
ISSN 2229-5518

Fig.5. HR-TEM micrograph and SAED pattern of -Al2O3
nanoparticles illustrates d-spacing.
Further characterization of platelet was applied using Selective Area Electron Diffraction (SAED), as shown in Figure.6. The regular squarepattern and strong diffraction spots indicate a single-crystal structure in the platelet and proof Rhombohedral crystalline structure obtained from XRD pattern for both samples.
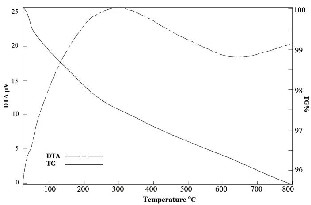
Thermal gravimetric and differential thermal analysis (TG/DTA) thermograph (Fig.6.) with soft slop and without sharp peaks proofs single phase and no impurity in sample.
Fig.6. TG/DTA graph of -Al2O3.
4 CONCOLUSION
Crystalline -Al2O3 platelets were synthesized by combustion of aluminum nitrate and urea mixture in aqueous media. High purity of -Al2O3 nanostructure obtained from combustion, illustrated in XRD and TG/DTA graph. TEM and SEM characterizations show platelet structure of product without requirement of annealing at high temperature.
REFERENCES
[1] Robert Ianos & et al, “The influence of combustion synthesis conditions on the -Al2O3 powder preparation,” J. Mater. Sci. vol.
44, pp.1016–1023, 2009.
[2] Fu-Su Yen & et al, “Characterization on microstructure homogeneity of θ-Al2O3 powder systems during phase transformation,” Key Eng. Mater. vol. 351, pp. 81-87, 2007.
[3] R. M’Saoubi & S. Ruppi, “Wear and thermal behaviour of CVD - Al2O3 and MTCVD Ti(C,N) coatings during machining,” CIRP
Annals – Manu. Tech. vol. 58, pp. 57–60, 2009.
[4] V. Naumann & et al, “Interface and material characterization of thin ALD-Al2O3 layers on crystalline silicon,” Energy Procedia vol.
27, pp. 312 – 318, 2012.
[5] B. Vermang & et al, “Integration of Al2O3 as front and rear surface passivation for large-area screen-printed p-type Si PERC,” Energy Procedia vol. 27, pp 325 – 329, 2012.
[6] Mohsen Hossein-Zadeh & et al, “Characterization of properties of
Al–Al2O3 nano-composite synthesized via milling and subsequent casting,” J. King Saud Uni. Eng. Sci. vol. 25, pp. 75–80,
2013.
[7] Guohua Zhang & et al, “The strain amplitude-controlled cyclic fatigue behavior of Al2O3 fiber reinforced Al–Si alloy compositeat elevated temperatures,” Progress in Natural Sci.: Mater. Int. vol. 22(2), pp153–159, 2012.
[8] Z. L. Xiao & et al, “Fabrication of Alumina Nanotubes and Nanowires by Etching Porous Alumina Membranes,” Nano Lett. vol. 2 (11), pp. 1293-1297, 2002.
[9] Yong Zhang & et al, “Selective Growth of -Al2O3 Nanowires and Nanobelts,” J. Nanomater. vol 2008, ID 250370, 2008.
[10] Hsing-I Hsiang & et al, “Synthesis of -Alumina Hexagonal Platelets Using a Mixture of Boehmite and Potassium Sulfate,” Amer. Ceram. Soci. J. vol. 90 (12), pp. 4070–4072, 2007.
[11] Zengzhi Zhang & et al, “Preparation and morphology of single crystal α-Al2O3 nanoparticles by combustion chemical deposition,” Procedia Eng. vol. 27, pp. 1284 – 1291, 2012.
[12] Päivikki Repo & et al, “Silicon Surface Passivation by Al2O3: Effect of ALD Reactants,” Energy Procedia vol. 8, pp. 681–687,
2011.
[13] R. M. Laine & et al, Nano--Al2O3 by liquid-feed flame spray pyrolysis, Nature Mater. vol. 5, pp. 710 – 712, 2006.
[14] T.Mimani & et al, “Solution combustion synthesis of nanoscale
oxides and their composites,” Mater. Phys. Mech. vol.4, pp. 134-
137, 2001.
[15] Singanahally T. Aruna & et al, “Combustion synthesis and nanomaterials,” Current Opinion in Solid State and Materials Science vol. 12, pp. 44–50, 2008.
[16] YI Jian-hong & et al, “Synthesis of crystalline γ-Al2O3 with high purity,” Trans. Nonferrous Meter. Soci. China. vol.19, pp. 1237−1242,
2009.
[17] Fu Su Yen & et al, “- to -phase transformation subsystem induced by -Al2O3 seeding in boehmite-derived nano-sized alumina powders,” J. Crys. Grow. vol. 249, pp. 283–293, 2003.
[18] ShaniKeysar & et al, “Heat Treatment of Alumina Aerogels,”
Chem. Mater. vol. 9, pp. 2464-2467, 1997.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013
ISSN 2229-5518
1596
[19]A. Janbey & et al, "A new chemical rout for the synthesis of nano
crystalline a-Ah03powder," J. Euro. Ceram. Soci. vol. 21, pp. 2285-
2286, 2011.
I£ER lb) 2013
http://www.ijserorq





