female hormones. The most potent form of estrogen is generally considered to be estradiol.
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 296
ISSN 2229-5518
Silveri Kalpana, Akondi Butchi Raju
St. Peters Institute of Pharmaceutical Sciences,Vidyanagar, Hanamkonda, Warangal-506001
Dr. Akondi Butchi Raju,
St. Peters Institute of Pharmaceutical Sciences, Vidyanagar, Hanamkonda, Warangal-506001
E-mail: drraju2020@gmail.com
The potential therapeutic utility of estrogens in schizophrenia is increasingly being recognised. The goal of the study was to assess the effect of genistein a phytoestrogen in ketamine induced rat model of schizophrenia. Schizophrenia was induced by administering ketamine 50mg/kg i.p. Behavioural models assessed were loco motor activity representing positive symptoms, forced swimming test representing negative symptoms, active avoidance test representing cognitive symptoms. Biochemical parameters like dopamine and acetyl cholinesterase were estimated in rat brain tissues. To assess the possible side effects of genistein on male fertility, andrological parameters of rats such as sperm count, motility, viability and histology of testis were also evaluated. Acute administration of ketamine produced hyperactivity response in loco motor activity test, when administered chronically enhanced the immobility period in animals during the forced swim test and reduced the number of avoidances in active avoidance test. In Genistein, standard (clozapine) and combination of both treated groups we found protective effect of the drugs. Out of three different regimes the combination of clozapine and genistein found to be better in normalizing the levels of various parameters conducted in the present study. So the potentiating effect of the clozapine and genistein drugs can be seen. Genistein a
phytoestrogen found to have no adverse effect on andrological parameters in male rats. Based on
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 297
ISSN 2229-5518
the results, genistein was found to be effective in all the symptoms of schizophrenia. Genistein in combination with antipsychotic drug clozapine found to have better protective effect. Genistein, a phytoestrogen has no effect on andrological parameters in male rats. So its use as an adjuvant therapy may be preferred along with standard drug treatment.
Schizophrenia is a chronic, severe, and disabling brain disorder that affects up to 1% of the population and makes it difficult for sufferers to think clearly, make decisions, and interact with people, as well as causing hallucinations, paranoia and many other symptoms. Schizophrenia affects around 24 million people worldwide as of 2011 (World Health Organization. 2011). Like many neurological diseases, the causes of schizophrenia are very complex, so while scientists have a basic idea of how the disease works, much about it is still a mystery.
The ‘estrogen hypothesis’ was derived from epidemiological, clinical and animal studies. Epidemiological studies (Hafner et al. 1993) have shown that women with schizophrenia present with first-episode psychosis, on average, about 5 years later than men with schizophrenia. Clinical studies reveal greater differences in the symptoms suffered, with men having more
negative symptoms of schizophrenia and women experiencing more affective and paranoid
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 298
ISSN 2229-5518
symptoms (Goldstein, 1988; Goldstein and Tsuang, 1990). Life-cycle studies have also shown that women are more vulnerable for either a first episode of psychosis or relapse of an existing illness at two major periods of hormonal change; firstly during the postpartum period and secondly during the menopause (Seeman, 1986, 1996). There have also been case reports of women whose schizophrenia symptoms were exacerbated at low estrogen phases of the menstrual cycle (Endo et al., 1978). Women are often more responsive to neuroleptic treatment than men. All of these trends seem to indicate that estrogen definitely has a delaying effect on schizophrenia. Estrogen is a generic term that encompasses several different types of similar
female hormones. The most potent form of estrogen is generally considered to be estradiol.![]()
Estrogen treatment reduced the sensitivity of the dopamine receptors, blockading them like neuroleptics
. A study found a connection between estrogen and NMDA receptors. They found
that NMDA-antagonists blocked the densifying effect of estrogen.Addition of estrogen to the brain cells increased the density of dendritic spines implying that estrogen works through the NMDA receptors
Modulates dopamine system
Modulates glutamate system
Overall neuroprotection.
Modulates dopamine synthesis
Restores mood,memory and cognition affected in schizophrenia.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 299
ISSN 2229-5518
The hypothesis of the present study was based on role of estrogens in schizophrenia. As discussed above in topic number “Estrogen and schizophrenia hypothesis”, the estrogen levels were found to be alarmingly low in patients with schizophrenia. Genistein being a phytoestrogen may be helpful in treating schizophrenia. So the genistein was evaluated for its activity in suitable animal models. The details of the current test drug “Genistein” was elaborated in the next chapter.
Albino wistar rats, (weight 150±20g), males were used for the present study. The animals were procured from Sanzyme limited, Hyderabad, India. They were housed in poly acrylic cages (38cm x 23cm x10cm) with not more than six animals per cage, at an ambient temperature of
25±2◦C with 12-h-light/12-h-dark cycle. Rats have free access to standard chow diet and water
ad libitum. The maintenance and the handling of animals were performed according to the guidelines and regulations of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi. The research protocols were approved by the Institutional Animal Ethical Committee (IAEC). Approval no: 12/SPIPS/IAEC/12.
Locomotor Activity (Chatterjee et al 2011)
Locomotor activity in rats was measured using the instrument actophotometer. The number of interruptions of the infrared beams along the spatial dimensions of the monitor by the animals was interpreted as horizontal activity counts. In this experiment the rats were divided into 6 groups, each group containing 6 animals (n=6). Prior to the experiment, both the control and the treatment group animals were habituated in the experimental instrument for 15 min and the basal activity scores were noted. Again after 24 h different groups were administered with following drugs, the vehicle (10 ml/kg, oral), clozapine (10mg/kg Oral), Genistein (12.5mg/kg Oral) were administered 60 min prior to the administration of ketamine (50mg/kg I.P). 30 min after ketamine administration each rat was tested for activity scores for 5 min.
Forced swimming test: (Chindo et al 2012)
Forced swimming test, is a measure of Despair behaviour. In brief, rat were placed individually in plastic cylinders (approx 45cm height, 21cm diameter) containing 20cm depth of water at 25 C.
After 5min, the animals were removed from water, dried and returned back to their home cages. They
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 300
ISSN 2229-5518
were again placed in the cylinder 24 h later and after the initial 1min acclimatization period, the total duration of immobility was measured for 5min. Rats which were floating motionless were considered to be immobile and the duration of swimming was measured.
Active avoidance test: (DAS et al 2003)
Active avoidance test helps to evaluate the associative learning of the animal.
Training for active avoidance test was conducted in Sidman jumping box (Elico, Chennai, India). It was divided in to two equal chambers (27×29×25cm) by Plexiglas partition, with a gate providing access to the adjacent compartment through a 14×17cm space. Prior to avoidance training each rat was habituated to the apparatus for 10 min. At the beginning of each session a rat was placed in the left compartment close to and facing the end wall. In each trial the animal is subjected to a light for
30 s followed by a sound stimulus for 10s. Immediately after the sound stimulus, the rat receives a single low intensity foot shock (0.5 mA; 3 s) through the grid floor. Each animal received a daily session of 15 trials with an inter-trial duration of 15 s for 5 days i.e., a maximum of 75 trials. Transfer time from one compartment to another, number of avoidances (after the stimulus either light alone or both light and sound) and escape (after the foot shock) response are recorded. The criterion for improved cognitive activity was taken as significant increase in the avoidance response on 5th session (retention) as compared to 1st session (training). All the behavioural models were carried out in a semi dark sound proof room in order to overcome external interferences in the experiment.
Estimation of Dopamine: Dopamine in the brain homogenates was measured using a method adopted from Schlumpf et al., 1974. On the day of estimation rats were sacrificed by cervical dislocation, whole brain was dissected out and separated. Weighed quantity 1.4 g of the brain tissue in 14ml of HCl- butanol was used for the homogenization. The sample was then centrifuged for 1 min at 2000 rpm. An aliquot of supernatant phase was removed and added to the centrifuge tubes containing 5ml of n- heptane and 625µl of 0.1 M HCl. After vigorous shaking for ten mins the tubes were centrifuged under the same conditions to separate aqueous and organic phases. Upper organic
phase was discarded and the aqueous 1.5 ml was used for the estimation of dopamine.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 301
ISSN 2229-5518
Acetyl cholinesterase enzyme activity was estimated by Ellman method. The rats were decapitated;
brains are removed quickly and placed in saline. The brain tissues are weighed and homogenized in
0.1M phosphate buffer (pH 8). 0.4ml aliquot of the homogenate is added to a cuvette containing 2.6ml phosphate buffer (0.1M, pH 8) and 100µl of DTNB. The contents of the cuvette are mixed thoroughly by bubbling air and absorbance is measured at 412nm in a spectrophotometer. When absorbance reaches a stable value, it is recorded as basal reading. 20µl of substrate i.e., acetylthiocholine is added and change in absorbance is recorded. Change in absorbance per minute is thus determined.
This study is aimed to evaluate the effect of genistein on male fertility by evaluating some andrological parameters of rats such as sperm count, motility, viability and morphology which are some of the indices that determine the ability of male to produce viable spermatozoa.
Immediately after killing, the epididymis was removed and trimmed of fat. Spermatozoa were obtained and prepared by method (kato et al 2002). Briefly caudal epididymis was minced in saline solution and incubated at 370C for 30 min to allow dispersion of spermatozoa.
The caudal sperm count test was performed according to (d’souza 2004). The spermatozoa count was obtained by counting the number of sperm cells in four WBC chambers using a neubauer’s slide.
Microcopic examinations of seminal smears staind with eosin were carried out to determine the % of sperm viability. Ratio of alive/ dead.
Testis of treated rats were taken and fixed in 10% neutral formalin solution. the fixed specimen were then trimmed, washed and dehydrated in ascending grads of alcohol. Specimens were cleared in
xylene, embedded in paraffin, sectioned at 4-6 microns thickness and stained.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 302
ISSN 2229-5518
The rats were divided into five groups, each group consisting of six animals. The rats were used for studying the protective effect of genistein against ketamine induced psychosis.
GROUP-I | Normal control |
GROUP-II | Positive control- treated with ketamine |
GROUP-III | Clozapine + ketamine |
GROUP-IV | Genistein + ketamine |
GROUP-V | Clozapine + Genistein + ketamine |
• Ketamine- 50mg/kg; intra peritoneal (i.p).
• Clozapine- 10mg/kg; per oral (p.o)
• Genistein- 12.5mg/kg; p.o
Clozapine dissolved in DMSO and genistein dissolved in sesame oil were administered
(p.o) to the rats according to the treatment protocol.
Groups III, IV, V were pre-treated with clozapine and genistein prior to the administration of ketamine. All groups of rats were assessed for the behavioural activities like locomotor activity, forced swimming, active avoidance test according to the given procedures and the observations are recorded. Finally the rats were sacrificed and the brain homogenates are used for the estimation of dopamine and Ach. Testis were collected from this cauda epididymis
was isolated and used for the estimation of sperm count, viability and motility.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 303
ISSN 2229-5518
Statistical analysis of all the obtained results was performed by one way ANOVA using graph pad prism software version 5.0 followed by Bonferroni’s multiple comparison test. All the results were expressed as mean±SEM. A probability of p<0.05 was considered as significant.
The experiments were conducted according to the above procedures and the observations are recorded. The results are drawn from observations and these results are discussed in the next chapters.
Animals were pre-treated with either Clozapine (10mg/kg) or Genistein (12.5mg/kg) or both through oral route 1hr prior to the ketamine administration. The locomotor activity was measured at 30 min after ketamine administration by using actophotometer. Each rat was tested for activity scores for 5 min.
Treatment Groups (n=6) | Horizontal activity counts Mean ± SEM |
Normal control | 100±12 |
Positive control - ketamine 50mg/kg | 450±30# |
Standard - clozapine 10mg/kg | 150±4.4* |
genistein - 12.5mg/kg | 260±16* |
clozapine(10mg/kg)+genistein(12.5mg/kg) | 91±5.2* |
*p<0.05 as compared to positive control; #p<0.05 as compared to normal control.
Effect of genistein on ketamine induced hyperlocomotor activity in rats. Each point is Mean ± S.E.M. Number of rats used per treatment for group are 6. One way ANOVA followed by Boenferoni’s multiple comparison test revealed significant difference between control and
various treatment groups.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 304
ISSN 2229-5518
LOCOMOTOR ACTIVITY

500
400
300
200
100
0
Ketamine significantly increased the locomotor activity. In Genistein, cloazipine and combination of both treated groups we found protective effect of the drugs. Out of 3 different regimes the combination of clozapine and genistein found to be better in maintaining the locomotion almost at normal levels. So the potentiation effect of the above drugs can be seen.
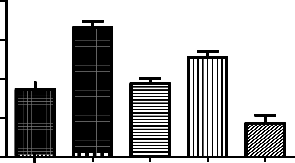
This is chronic study for about 10 days. Animals were pre-treated with either Clozapine (10mg/kg) or Genistein (12.5mg/kg) or both through oral route 1hr prior to the ketamine administration for 8 days, then on 9th day rats were placed individually in plastic cylinders containing water. After 5min, the animals were removed from water, dried and returned back to the cages. They were again placed in the cylinder 24 h later and after the initial 1min acclimatization period, the total duration of immobility was measured for 5min.
Treatment Groups (n=6) | Immobility duration (secs) |
Normal control | 87±8.3 |
Positive control - Ketamine 50mg/kg | 170±8.5# |
Standard - Clozapine 10mg/kg | 94±6.9* |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 305
ISSN 2229-5518
Genistein 12.5mg/kg | 130±7.6* |
Clozapine10mg/kg+Genistein12.5mg/kg | 43±11* |
*p<0.05 as compared to positive control; #p<0.01 as compared to normal control
Effect of genistein on ketamine induced immobility in rats. Each point is Mean ± S.E.M. Number of rats used per treatment for group are 6. One way ANOVA followed by Boenferoni’s multiple comparison test revealed significant difference between control and various treatment
groups.
200
FORCED SWIM TEST
150
100
50
0
Ketamine significantly increased the immobility duration. In Genistein, cloazipine and combination of both treated groups we found protective effect of the drugs. Out of 3 different regimes the combination of clozapine and genistein found to have better effect. The potentiation effect of the above drugs can be seen.
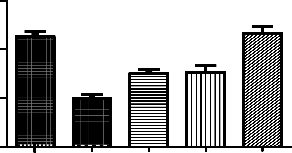
It’s also a chronic study for period of 10 days, for successful induction memory impairment by ketamine. Rats were pretreated with drugs for 10 days. From the 5th day each animal received a daily session of 15 trials with an inter-trial duration of 15 s for 5 days i.e., a
maximum of 75 trials in shuttle box. The number of avoidances was recorded.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 306
ISSN 2229-5518
Treatment Groups (n=6) | No of avoidances |
Normal control | 11±0.49 |
Positive control - Ketamine 50mg/kg | 5.0±0.37# |
Standard - Clozapine 10mg/kg | 7.5±0.43* |
Genistein 12.5mg/kg | 7.6±0.67* |
Clozapine10mg/kg+Genistein12.5mg/kg | 12±0.67* |
*p<0.05 as compared to positive control; #p<0.01 as compared to normal control
Effect of genistein on ketamine induced cognitive impairment in rats. Each point is Mean
± S.E.M. Number of rats used per treatment for group are 6. One way ANOVA followed by
Boenferoni’s multiple comparison test revealed significant difference between control and various treatment groups.
ACTIVE AVOIDANCE TEST
15
10
5
0
Ketamine significantly decreased the number of avoidances. In Genistein, cloazipine and combination of both treated groups we found protective effect of the drugs. Out of 3 different
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 307
ISSN 2229-5518
regimes the combination of clozapine and genistein found to be better in increasing the number of avoidances almost at normal levels. The potentiation effect of the above drugs can be seen.
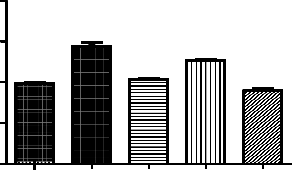
Dopamine levels in rat brain were estimated by using photoflourimeter. The test and the standard serial dilutions of dopamine were recorded against blank at excitation and emission wavelength 340- 580.
Treatment Groups (n=6) | Concentration of dopamine(ng/mg) |
Normal control | 39±0.52 |
Positive control - Ketamine 50mg/kg | 58±2.1# |
Standard - Clozapine 10mg/kg | 41±0.52* |
Genistein 12.5mg/kg | 51±0.52* |
Clozapine10mg/kg+Genistein12.5mg/kg | 36±1.0* |
*p<0.05 as compared to positive control; #p<0.01 as compared to normal control
Effect of genistein on ketamine induced alteration of dopamine levels in rat brains. Each point is Mean ± S.E.M. Number of rats used per treatment for group are 6. One way ANOVA followed by Boenferoni’s multiple comparison test revealed significant difference between
control and various treatment groups.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 308
ISSN 2229-5518
DOPAMINE ESTIMATION
80
60
40
20
0
Ketamine significantly increased the dopamine levels in rat brains. In Genistein, cloazipine and combination of both treated groups we found protective effect of the drugs. Out of
3 different regimes the combination of clozapine and genistein found to be better in decreasing the dopamine almost to normal levels. The potentiation effect of the above drugs can be seen.
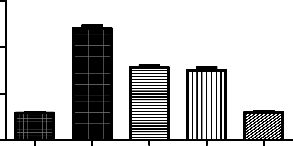
Treatment Groups (n=6) | Concentration of AchE µ moles/ml/min/mg protein |
Normal control | 118±1.76 |
Positive control - Ketamine 50mg/kg | 480±12.5# |
Standard - Clozapine 10mg/kg | 312±8.36* |
Genistein 12.5mg/kg | 302±10.3* |
Clozapine10mg/kg+Genistein12.5mg/kg | 119±3.76* |
*p<0.05 as compared to positive control; #p<0.01 as compared to normal control
Effect of genistein on ketamine induced alteration of dopamine levels in rat brains. Each point is Mean ± S.E.M. Number of rats used per treatment for group are 6. One way ANOVA
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 309
ISSN 2229-5518
followed by Boenferoni’s multiple comparison test revealed significant difference between
control and various treatment groups.
600
400
200
0
Ketamine significantly increased the AchE levels in rat brains. In Genistein, cloazipine and combination of both treated groups we found protective effect of the drugs. Out of 3 different regimes the combination of clozapine and genistein found to be better in decreasing the AchE levels almost to normal levels. The potentiation effect of the above drugs can be seen.
Groups | Normal control | Genistein | Clozapine +genistein |
Sperm count (106 cells) | 63.33±2.6 | 62±2.06 | 64±1.07 |
Motility | 79±5.70 | 72±1.76 | 76±1.45 |
Viability | 66.33±0.69 | 64±2.10 | 70±4.02 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 310
ISSN 2229-5518
No significant difference observed in sperm count, motility, viability in different treatment groups.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 311
ISSN 2229-5518
Genistein isoflavone, a major constituent of soybeans doesn’t produce any adverse effect on male reproductive organs. There is no significant difference observed in sperm count, motility, and viability when compared with normal control group.
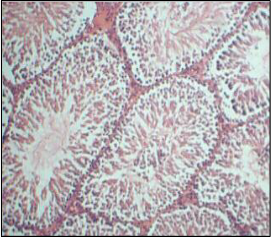
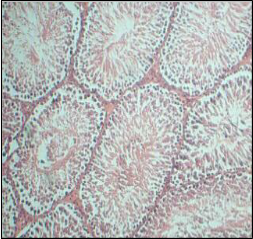
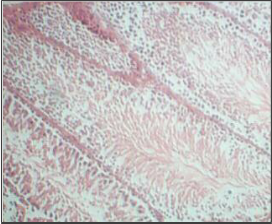
Histopathology of testis of individual genistein treated and combination of genistein & clozapine treated groups doesn’t showed any significant difference when compared with control group. All groups showed normal histological arrangements of blood vessels, Leydig cells and a seminiferous tubule with lumen.
The results observed here are interpreted and discussed in the next chapter.
Schizophrenia is a chronic debilitating psychiatric disorder affecting as many as 1% of the population worldwide (Fauci 2008).
The clinical features of the heterogeneous disorder schizophrenia usually appear in late adolescence or early adulthood, and can be divided into positive, negative and cognitive symptoms. Delusions (paranoia, false beliefs), hallucinations (visual and auditory), and thought disturbances are classified as positive symptoms, whereas negative features include depression, withdrawal from social contacts, incapability to feel pleasure (anhedonia) and flattening of emotional responses. Cognitive defects involve deficits in attention and memory. (Rajiv Tendon et al 2009)
The residual symptoms especially negative and cognitive symptoms are closely correlated with the degree of disability than the positive symptoms. Atypical antipsychotic medication provides hope in the management of negative symptoms, but the success remains limited. (Buckley et al. 2007)
The dopaminergic hypothesis came about from the observation that drugs that antagonized dopamine were found to be effective in the treatment of schizophrenia. Dopamine
system has been proposed to account for the positive and negative symptoms of schizophrenia
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 312
ISSN 2229-5518
that emerge during adolescence. Dopamine antagonists are effective only in treating positive symptoms associated in schizophrenia.
The estrogen hypothesis suggests that estrogen provides protection from the development of Schizophrenia and decreased the severity of negative symptoms (Seeman 1982; Seeman& Lang 1990). Preclinical data supports the involvement of estrogen in the regulation of several neurotransmitter systems (Dopamine, Serotonin, Noradrenalin and Glutamate) (McEwen 2002; Cyr et al. 2002). Beside direct influence on neurotransmission, estrogen may play a role in Schizophrenia by susceptibility gene regulation (Olsen et al. 2008). It involves estrogen effects variety processes during the brain development includes neuronal differentiation, survival and excitability (Boulware & Mermelstein 2005; Garcia-Segura et al. 2001). Many studies have indicated that estrogen replacement therapy has a beneficial effect on cognition function.
The use of estrogen as adjuvant treatment appears promising, but its use in long-term treatment has the disadvantage of the potential negative effect estrogen can have on breast and uterine tissue. (Chua et al 2005; Chlebowski et al 2009) Estrogen non-selectively acts on ER- alpha receptors are associated with the traditional female sexual effects and are found in the uterus, testis and adrenal gland.
Soy isoflavones, which are referred to as phytoestrogens and include genistein, can bind to estrogen receptors (ERs) and affect estrogen-mediated processes (Molteni et al 1995). Pan et al 2000) Genistein is a relatively selective estrogen receptor β agonist (Tzagarakis-Foster; Lomri
& Leitman 2001). ER-beta is present in more diverse parts of the body, including the brain.
The goal of the study was to assess the efficacy of genistein using ketamine-induced working model in rat with respect to some selected behavioural phenotypes that correlate with certain sections of symptoms observed in schizophrenia. The present study was also aimed at evaluation of the neurochemical changes in the brain tissues which are implicated in the pathophysiology of schizophrenia. To assess the possible side effects of genistein on male fertility, andrological parameters of rats such as sperm count, motility, viability and morphology
were also evaluated.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 313
ISSN 2229-5518
Ketamine was a proven inducing agent of schizophrenia by blockade of the NMDA receptor channel complex by non-competitive antagonism induces symptoms commonly seen in schizophrenia (Krystal et al. 1994). Sub-anaesthetic doses of NMDA receptor antagonists, such as phencyclidine (PCP), MK-801 and ketamine, were reported to induce a wider spectrum of behavioural responses that encompass positive, negative, and cognitive schizophrenia-like symptoms in healthy human volunteers (Javitt and Zukin, 1991) and rodents (Chatterjee et al.
2011). In the present study the behavioural models such as locomotor activity, forced swimming test, active avoidance test were conducted and Acetylcholinesterase enzyme and dopamine levels in brain tissue were estimated.
Locomotor activity is a measure of the positive symptoms in schizophrenia. Positive symptoms include aggressive and stereotypy behaviour. Results in the present study indicate that acute treatment of ketamine (50 mg/kg, i.p.) produced hyper locomotor response. The hyperlocomotory activity observed here are believed to be the result of dopamine agonistic action induced by ketamine. The locomotor activity was almost normal when genistein was given along with clozapine in ketamine received rats and it’s an indication of protection from the occurrence of positive symptoms in schizophrenia. But genistein alone was not effective on par with combination of genistein and clozapine. We found this phenomenon was in agreement with the published literature. The past literature suggests, A 28- day clinical study has found that addition of 100 mcg of transdermal estradiol provided better clinical improvement of psychotic symptoms of hallucinations, thought disorder and delusion in women patients (Kulkarni et al
2001).
Forced swimming test is a suitable animal model that reflects negative symptoms of schizophrenia. Depression is one of the major negative symptoms of schizophrenia. Forced swim-induced immobility in rodents is an acceptable animal model of schizophrenia that reflects a state of despair in the rat and the reduction in the immobility time serves as the index of antidepressant activity.
In our study chronic administration of ketamine for 10 days induced enhancement of the immobility duration of rats in forced swimming test. The reduction in immobility duration was almost normal when genistein was given along with clozapine in ketamine received rats and it’s
an indication of protection from the occurrence of negative symptoms in schizophrenia. But
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 314
ISSN 2229-5518
genistein alone was not effective on par with combination of genistein and clozapine. This phenomenon was in agreement with the previous published literature, and it suggests that, raloxiphene, a selective estrogen receptor modulator, when given along with antipsychotic treatment in postmenopausal women with schizophrenia who are exhibiting negative symptoms significantly reduced both positive and negative symptoms. (Judith Usall et al 2010). The enhancement of immobility after chronic administration of phencyclidine, ketamine has been used previously as a model for the negative symptoms of psychosis, such as flattening of affect and avolition (Noda et al 1995; Chatterjee et al 2011).
Cognitive impairments such as deficits in attention, executive function, working (short- term) memory, and long-term memory, are core symptoms in patients with schizophrenia. Among these, learning and memory impairments are known to be particularly severe, and they are suggested to be major determinants of the amount of disability patients with schizophrenia. The active avoidance test has been used to evaluate the effects of antipsychotic drugs on learning and memory function. Genistein improved the ketamine induced cognitive symptoms in schizophrenic rats in our study. The increase in the number of avoidances was normal when genistein was given along with clozapine in ketamine received rats and it’s an indication of protection from the occurrence of cognitive symptoms in schizophrenia. Both genistein and clozapine equally increased the number of avoidances. This phenomenon was in agreement with the previous published literature, and it suggests that Genistein improves memory of Ab-injected rats in (M. Bagheriet al. 2011) study. Acute and chronic ketamine administration, differentially and site specifically, modulated the levels of acetylcholine, dopamine, serotonin and noradrenaline. (Chatterjee et al. 2012)
Acetylcholine plays a critical synaptic role in the initial stages of memory formation (Hasselmo, 2006). Ketamine blocks nicotinic cholinergic receptors (Scheller et al, 1996) and there by suppresses glutamate release. Blockade of these receptors, induces increased ACh release activating cholinesterase enzyme which, in turn, interferes with hippocampal memory formation. Genistein individually reduced the Ache levels and in combination with clozapine
produced potentiating effect.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 315
ISSN 2229-5518
Increased locomotor activity was found in locomotor activity test. It has been reported that dopaminergic pathways are critical for the control locomotor activities. Ketamine increased the dopamine levels (Chatterjee et al 2012). Dopamine levels were almost normal when genistein was given along with clozapine in ketamine received rats and it’s an indication of protective effect. But genistein alone was not effective on par with combination of genistein and clozapine.
This phenomenon was in agreement with the previous literature and it suggests Animal models have found that estrogen can inhibit the actions of dopamine (Hafner et al. 1991). Various animal studies found that estrogen treatment has been shown to reduce the dopamine concentration in the striatum and modulate sensitivity as well as the number of dopamine receptors (Foreman and Porter, 1980; Koller et al. 1980; Gordon et al. 1980; Dupond et al. 1981; Bedard et al. 1984). The effect of genistein on male fertility is assessed by evaluating some andrological parameters of rats such as sperm count, motility, viability and histology of testis.
Genistein isoflavone, a major constituent of soybeans has no adverse effect on male reproductive organs. There is no significant difference observed in sperm count, motility, and viability in genistein and combination of genistein and clozapine groups when compared with normal control group. Histopathology of testis of individual genistein treated and combination of genistein & clozapine treated groups doesn’t showed any difference when compared with control group. All groups showed normal histological arrangements of blood vessels, leydig cells and a seminiferous tubule with lumen.
This phenomenon was in agreement with the previous literature and it suggests as, A
2010 meta-analysis of fifteen placebo-controlled studies said that "neither soy foods nor isoflavone supplements alter measures of bio available testosterone concentrations in men"(Hamilton-Reeves et al. 2010). Furthermore, isoflavone supplementation has no effect on sperm concentration, count or motility, and leads to no observable changes in testicular or ejaculate volume (Dabrowski, Waldemar 2004, Mitchell et al 2001). This makes sense, as estradiol, the endogenous estrogen agonist, and a metabolite of testosterone, inhibits sperm cell apoptosis.
Schizophrenia is a chronic and disabling mental illness affecting millions of people worldwide. The symptoms of schizophrenia are classified into positive, negative and
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 316
ISSN 2229-5518
cognitive symptoms. New receptor targets and drugs have being evaluated for addressing the multifaceted syndrome of schizophrenia. “Estrogen hypothesis” of schizophrenia posits that estrogen has a protective effect in women who are susceptible to presenting with this illness. In the present study genistein being a phytoestrogen is evaluated for its effect in schizophrenic rats. The results suggest that treatment with genistein is effective in all the symptoms of schizophrenia. Genistein was found to modulate the levels of dopamine and acetyl cholinesterase enzyme. Genistein in combination with antipsychotic drug clozapine found to have better protective effect. Genistein, a phytoestrogen doesn’t produce any adverse effect on andrological parameters in male rats. Its use as an adjuvant therapy can be preferred along with standard drug treatment.
1. Anna Elisa Valsecchi, Silvia Franchi, Alberto Emilio Panerai, Alice Rossi, Paola Sacerdote, Mariapia Colleoni. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol 2001;650:694-702.
2. Baldersseran. Do central adrenergic action contribute to the atypical properties of clozapine. Brit J psychiat 1992;17:12-16.
3. Boulware, M.I., Mermelstein, P.G., (2005) The influence of estradiol on nervous system function. Drug News Perspect, 18, 631–637.
4. Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr Scand.
2007;115:93-100.
5. Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on the formation of 3- methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Tox
1963;20:140-44.
6. Carlsson A, Lindqvist M, Magnusson T. 3, 4-Dihydroxyphenylalanine and 5- hydroxytryptophan as reserpine antagonists. Nature 1957;180:1200.
7. Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HA.
Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 317
ISSN 2229-5518
of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci
1999;51:236-44.
8. Catalyst Treatment Advocacy Center. Schizophrenia Facts. [updatedNovember 23,
1999].Available from: www.psychlaws.org/General%20resources/Facts.htm.
9. Chlebowski RT, Kuller LH, Prentice RL, et al; WHI Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009; 360:573-87.
10. Chua WL, de IZquierdo SA, Kulkarni J, et al. Estrogen for schizophrenia. Cochrane
Database Syst Rev. 2005;19:CD004719.
11. Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein and their β- glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agr food chem 1993;41(11):1961–67.
12. Coyle J.T., Tsai G., Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann ny acad sci 2003;1003:318–27.
13. Creese I, Burt, D.R., Snyder S.H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976;192:481-83.
14. Cyr, M., Calon, F., Morissette, M., & Di, P.T. Estrogenic modulation of brain activity:
implications for schizophrenia and Parkinson's disease. J Psychiatry Neurosci.
2002;27:12–27.
15. Daly SA, Waddington JL. Two directions of dopamine D1/D2 receptor interaction in studies of behavioural regulation a finding generic of four new selective dopamine D1 receptor. Eur J Pharmacol 1992;213:251-58.
16. Daly, S.A., Waddington , J.L. Two directions of dopamine D1/D2 receptor interaction in studies of behavioural regulation a finding generic of four new selective dopamine D1 receptor. Eur J Pharmacol 1992;213:251-258.
17. Das A, Dikshit M, Nath C, Singh HK. Evaluation of effect of scopolamine on stages of active avoidance learning in rats. IJP 2003;35:47-50.
18. Dean, Brian et al. Changes in Serotonin 2A and GABA Receptors in Schizophrenia: Studies on the Human Dorsolateral Prefrontal Cortex. J neurochem 1999;72:512-19.
19. Ek O et al. In vivo toxicity and pharmacokinetic features of B43(Anti-CD19)-Genistein
immunoconjugate. Leuk Lymphoma 1998;30:389-94.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 318
ISSN 2229-5518
20. Garcia-Segura, L.M., Azcoitia, I., & DonCarlos, L.L. Neuroprotection by estradiol. Prog
Neurobiol 2001;63,29–60.
21. Gary E. Duncan, Brian B. Sheitman, Jeffery A. Lieberman An integrated view of pathophysiological models of schizophrenia Brain Research Reviews 1999;29:250–264.
22. Goff D.C, Coyle R.J. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001;158:1367-68.
23. Hafner, Heinz et al. Causes and Consequences of the Gender Difference in Age at Onset of Schizophrenia. Schizophrenia Bulletin 1999;24:134-37
24. Heinz Häfner, Wolfram an der Heiden. Epidemiology of Schizophrenia. Can J Psychiatry
1997;42:139–51.
25. Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatr Scand
2004;109:269–74.
26. Javitt D. C. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate
Receptors, and Dopamine-Glutamate Interactions. Int Rev Neurobiol 2007;78:69-108.
27. Javitt, D.C., Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia.
Am J Psychia 1991;148:1301-08.
28. Jentsch J.D., Roth R.H. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacol 1999;20:201-25.
29. Jorgensen M, Vendelbo B, Skakkeaek NE, Leffers H. Assaying estrogenicity by quantitating the expression levels of endogenous estrogenregulated genes. Environ. Health Perspect 2000;108:403–12.
30. Kaufman PB, Duke JA, Brielmann H, Boik J, Hoyt JE. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health. J Altern Complement Med 1997;3(1):7–12.
31. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther 2003;97(2):153–79.
32. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg
B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol 1998;139(10):4252–63.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 319
ISSN 2229-5518
33. Kulkarni J, Riedel A, de Castella AR, et al. Estrogen- a potential treatment for schizophrenia. Schizophr Res 2001;48(1):137–44.
34. Lahti A.C., Weiler M.A., Tamara Michaelidis B.A., Parwani A., Tamminga C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacol.
2001;25(4):455–67.
35. Leclerq G, Heuson JC. Physiological and pharmacological effects of estrogens in breast cancer. Biochem Biophys Acta 1979;560:427–55.
36. Lieberman J.A, Kane, J.M, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacol 1987;91:415-33.
37. Li-Xing Liu A, Wen-Fang Chen A, Jun-Xia Xie A, Man-Sau Wong. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res 2008;60:156–61.
38. Margret schlumpf et al. A fluorometric micro method for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue. Biochem pharmacol 1974;23:2337- 46 .
39. McAllister, C.G., et al. Increases in CSF levels of Interleukin-2 in Schizophrenia: Effects of recurrence of psychosis and medication status. Ame J Psychia 1995;152:64-78.
40. McEwen, B. Estrogen actions throughout the brain. Recent Prog Horm Res 2002;57:357–
84.
41. Meador-Woodruff, Dr. Jones H. Schizophrenia. Meador-Woodruff Laboratories. www.- personal.umich.edu/~jimmw/. November 21, 1999.
42. Messinger Y, et al. In vivo toxicity and pharmacokinetic features of B43 (anti-CD19)- genistein immunoconjugate in nonhuman primates. Clin Cancer Res 1998;4:165-70.
43. Miodini P, Fioravanti L, Di Fronzo G, Cappelletti V. The two phytooestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. Cancer
1999;80:1150–55.
44. Munro IC et al. Soy isoflavones: a safety review. Nutr Rev 2003;61(1):1–33.
45. Neuroscience Institute of Schizophrenia and Allied Disorders. Current Schizophrenia
Research. November 21, 1999. Aailable from:
www.nisad.org.au/sitemap/info/info_frame.html.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 320
ISSN 2229-5518
46. Olsen ,L., Hansen, T., Jakobsen, K.D., Djurovic, S., Melle, I., Agartz, I., Hall, H., Ullum, H., Timm, S., Wang, A.G., Jonsson, E.G., Andreassen, O.A. & Werge, T. The estrogen hypothesis of schizophrenia implicates glucose metabolism.Nutr Rev 2005;45:78-84.
47. Pachaiappan Pugalendhi, Shanmugam Manoharan, Nagarethinam Baskaran, Madhavan R. Nirmal. Effects of genistein and daidzein, in combination, on the expression pattern of biomolecular markers (p53, PCNA, VEGF, iNOS, Bcl-2, and Bax) during 7,12- dimethylbenz(a)anthracene (DMBA) induced mammary carcinogenesis in Sprague- Dawley rats. Int J Biol Med Res 2010;1(4):264-71.
48. Picchioni MM, Murray RM. Schizophrenia. BMJ 2007;335(7610):91–95.
49. Rajiv Tandon, Matcheri S. Keshavan, Henry A. Nasrallah. Schizophrenia facts.
Schizophrenia, “Just the Facts” What we know in 2008.2. Epidemiology and etiology. Schizophrenia Research 2008;102:1–18
50. Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 7th edition. Edinburgh:
Churchill livingstone 2011:254-55.
51. Schizophrenia. World Health Organization. 2011. Retrieved February 27, 2011.
52. Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976;261:717-19.
53. Seeman, M.V., & Lang, M. The role of estrogens in schizophrenia gender differences.
Schizophr Bull 1990;16:185-94.
54. Seeman. M.VGender differences in schizophrenia. Can J Psychiatry. 1982;27(2), 107-12.
55. Sesack S. R, Carr D. B. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav 2002;77:513-17.
56. Sumner BE, Fink G. Estradiol-17b in its positive feedback mode significantly increases
5-HT2a receptor density in the frontal, cingulate and piriform cortex of the female rat. J
physiol 1995;483:52,
57. Sunyer Teresa et al. Estrogen’s bone-protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J Clin Invest 1999;103:10
58. Tamminga C.A., Holcomb H.H., Gao X.M., Lahti A.C. Glutamate pharmacology and the treatment of schizophrenia: current status and future directions. Int Clin
Psychopharmacol 1995;3:29-37.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 321
ISSN 2229-5518
59. The ICD-10 Classification of Mental and Behavioural Disorders. World Health
Organization. 26.
60. U.S. Pharmacist. The New Science of Estrogen Receptors. http://www.uspharmacist.com/NewLook/Ce/er/lesson.htm. November 18, 1999.
61. Van Os J, Kapur S. Schizophrenia. Lancet 2009;374(9690):635–45.
62. Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci
2001;21:1809-18.
63. Walter ED. Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans. J Am Chem Soc 1941;62(12):3273–76
64. Weickart Cynthia, Weinberger Daniel. A candidate approach to defining developmental pathology in schizophrenia. Schizophrenia Bulletin 1998;24(2):7-12.
65. Wooley, Catherine, and McEwen, B.S. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci
1994;14:509-12.
66. Xijin Wang, Shengdi Chen CA, Guozhao Main Ye, Guoqiang Lu. Genistein protects dopaminergic neurons by inhibiting microglial activation. LippincottWilliams &Wilkins
2005;16:3-28.
67. Yoon-Bok Lee, Hyong Joo Lee T, Heon Soo Sohn. Soy isoflavones and cognitive function. J Nutr Biochem 2005;16:641–49.
IJSER © 2013 http://www.ijser.org