

International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1693
ISSN 2229-5518
Abstract: This study was designed to evaluate the potential uses of a natural biomaterial-Chitosan as plant growth promoter and anti-fungal agent. Chitosan treated with Co-60 gamma radiation at different doses, were used to evaluate the efficiency of irradiated chitosan on tea plants. The aim of this study was to measure the effects of various concentrations of irradiated chitosan (300, 500, 1000 ppm) solutions in order to get best response of tea plants in terms of various attributes. Chitosan were applied through foliar spraying at 7 days interval. The growth attributes like- total number of buds, fresh and dry weight of buds, average leaf area, and weight per bud and anti-fungal activities were determined at 10 days interval after foliar application of biomaterials for 11 weeks. The results showed increase in productivity (about 38%, based on fresh weight of tea leaves) and it reduced the total fungal count dramatically (more than 100 times in contrast with the control).
Keywords: Anti-fungal Activity, Chitosan, Irradiation, Plant Growth Promoter, Tea plants
—————————— ——————————
In recent years, there is a trend in agriculture to limit the usage of chemical compounds and to encourage the use of biomaterials. Biomaterials are new promising materials that possess important properties like biodegradability or lack of toxicity. Radiation processing of biomaterials is an area of current research for development of new applications [1, 2, 3, 4]. Different living organisms like shrimps, crabs, brown algae etc. produce some biomaterials in their body to tolerate in adverse environmental conditions which have the plants growth promoting and anti-microbial capacity [5, 6]. The advantages of using these biomaterials are that, they are naturally available, cheap and have no destructive effect on overall environment including plants and animals which may be occurred in case of application of chemical fertilizers and pesticides. In this experiment chitosan was used to increase tea productivity which is naturally occurring biomaterial extracted from crustacean shells and sea weeds respectively [7].
Chitosan is a linear polysaccharide derived from chitin, a major component of the shell of crustaceans and the second most abundant biopolymer in nature next to cellulose and is commercially available [8]. It has the potential in agriculture with regard to controlling plant diseases and promoting the plant growth [9, 2]. These molecules were shown to display toxicity and inhibit fungal growth and development [10].
Irradiation can modify the viscosity, molecular weight, hydrophilic and mechanical properties of chitosan resulting enhanced properties. Previous work focused on the field test of chitosan as growth promoters for red chili, potato, and carrot plants [11]. This treatment also increases the productivity of soybean (using Mitani and Rajabasa varieties) in about 40% than control [12]. Growth-promotion effect of radiation degraded alginate on tea has also been studied in Vietnam, which indicated that a 100 ppm radiated alginate causes an increase in the bud weight almost 35% [12]. This research work is concerning on
————————————————
• aDepartment of Food Engineering and Tea Technology, Shahjalal University of Science and Technology,
Sylhet-3114, Bangladesh.
• bIntitute of Radiation and Polymer Technology, Bangladesh Atomic Energy Commission,
P. O. Box-3787, Dhaka, Bangladesh
*Corresponding Author: M. A. Khan
Email: makhan.inst@gmail.com
the effect of radiation processed chitosan on tea plants at different radiation doses and concentrations and make a comparative study among treated and untreated plants.
Extraction of chitosan from shrimp shells and preparation of 2% solution in 2% acetic acid were done at the laboratory of Institute of Radiation and Polymer Technology of Bangladesh Atomic Energy Research Establishment according to standard procedure [13].
Chitosan solution was then irradiated by gamma radiation emitted from a Co-60 source at different doses for degradation with a dose rate of
4.5 kGy per hour.
The relative viscosity of each sample solution (before and after radiation) was measured with an Ubbelohde glass capillary viscometer at 250C.
The irradiated solutions of chitosan were diluted at different concentration level before spraying. Each solution was diluted to 300,
500, and 1000 ppm using double distilled water. Hence, radiation doses used for chitosan solutions were 0(un-irradiated), 20, 30, 35, 40, and 50 kGy. Thus, the total numbers of solutions prepared from 2% Chitosan solution were 18 (6×3=18). The solutions of different doses were applied at the rate of 200mL- per plot area of tea plantation by using normal hand sprayer. Foliar applications of biomaterials were done at every 7 days interval.
The fresh tea buds for different treatment were collected from plantation plots. 8 different samples were selected- which include 1 control, 7 samples from plants treated with 1000 ppm chitosan solution at different radiation doses. To prepare sample, 0.89% saline water was prepared from pure sodium chloride. Then 9 ml of saline water was poured into 20 ml size conical flask. Then 1 g of tea leaves from each sample was soaked into corresponding conical flask followed by shaking to disperse fungus into saline water. Then 0.1mL (100 µL) saline water was inoculated onto PDA media and spreaded using L- shaped spreader. After spreading, the inoculated PDA petri plate was
incubated in incubator for three days at 300C. The enumeration of fungal count was done by colony counter [14].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1694
ISSN 2229-5518
The data was analyzed statistically using SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA). Mean and Standard Deviation were measured by Non Parametric Kruska-W allis One W ay ANOVA Test. The least significant difference was calculated for the significant data at P < 0.05.
The results obtained from this measurement are shown in table 01. It was found that the viscosity of chitosan solutions was decreased with the decrease in radiation doses.
Table 01: Changes in viscosity of chitosan solution with different radiation doses
Radiation Doses | Control | 20 kGy | 30 kGy | 35 kGy | 40 kGy | 50 kGy |
Viscosity (centipoise) | 2.0989 | 1.1522 | 1.1078 | 1.1008 | 1.1073 | 1.0878 |
The results of field experiments are shown in figure 02. The figures represent the effects of irradiated chitosan solution on tea plants.

Figure 01: Tea plants treated with chitosan solution in contrast with control
It exhibits that, the effectiveness of chitosan solution has increased with the increase in radiation doses up to 40 kGy, but it reduces when irradiated at 50 kGy. It also shows that, bud numbers were maximum in case of 500 ppm concentration, but it reduced at both very low (300
ppm) and very high (1000 ppm) concentration. Here it needs to be mentioned that, although different treatment showed enhanced properties on tea productivity, but statistically it could not proved.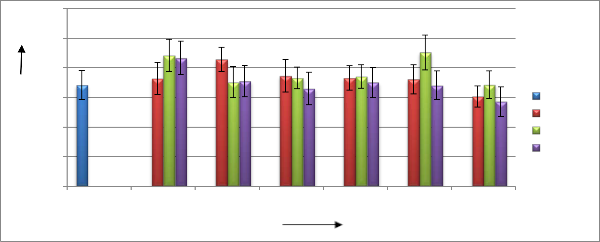
120
100
80
60 Control
300 ppm
40 500 ppm
1000 ppm
20
0
Control Un-irradiated 20 kGy 30 kGy 35 kGy 40 kGy 50 kGy
Treatment
Figure 02: Effects of radiation processed chitosan solution on number of buds of tea plants
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1695
ISSN 2229-5518
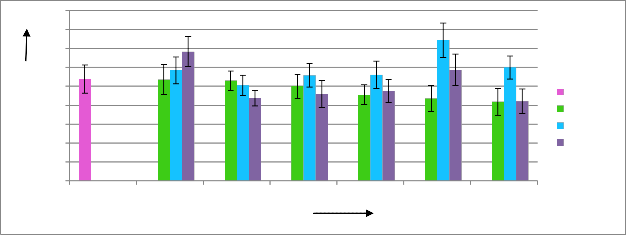
From figure 03, it is found that, the highest amount of fresh tea leaves were obtained from tea plants treated with the chitosan solution of 500 ppm concentration at 40 kGy radiation doses. There were about 37.14 g of fresh tea leaves was found at an average, which indicated that, tea plants treated with chitosan solution at a concentration of 500 ppm with 40 kGy radiation doses gave an increase of 38.16% fresh weight
45
40
35
30
25
20
15
10
5
0
of buds. Chitosan solution at 300 ppm concentration produced highest tea leaves when it was un-irradiated. At 1000ppm concentration also it gives best productivity when it was un-irradiated. The results were gradually increased up to 40 kGy, then at 50 kGy radiation doses it decreased. Fresh weight of tea buds were also decreased at both low and higher concentration.
Control
300 ppm
500 ppm
1000 ppm
Control Un-irradiated 20 kGy 30 kGy 35 kGy 40 kGy 50 kGy
Treatment
Figure 03: Effects of radiation processed chitosan solution on fresh weight of tea leaves
Like fresh weight of tea leaves highest dry leaves were obtained from solution treated with 40 kGy radiation doses at a concentration of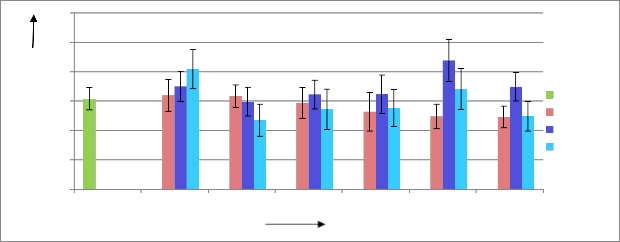
500ppm, which is about 42.29% higher than the control. In case of
300ppm and 1000 ppm solution highest dry leaves were obtained when the solutions were un-irradiated. Figure 04 illustrates the effects of radiation processed Chitosan solution on weight of dry tea leaves.
12
10
8
6 Control
300 ppm
4 500 ppm
1000 ppm
2
0
Control Un-irradiated 20 kGy 30 kGy 35 kGy 40 kGy 50 kGy
Treatment
Figure 04: Effects of radiation processed chitosan solution on weight of dry tea leaves
Table 02 shows that, the average leaf area of tea buds found-was
15.48 cm2. The maximum leaf area (21.258 cm2) was obtained from plants treated with 500ppm chitosan solution irradiated at 40 kGy dose.
It shows that, a 37.32% increase in the leaf area was obtained. At
300ppm concentrated solution maximum leaf area was obtained at 30 kGy radiation doses. In case of 1000ppm concentrated solution maximum leaf area was found when the solution was treated at 40 kGy radiation dose.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1696
ISSN 2229-5518
The maximum weight of tea bud was obtained from 50 kGy radiation doses, and at 500 ppm concentrated solution. It was found that, about
11.12% increase in the average weight of bud was obtained from plants treated with irradiated Chitosan. At 300ppm concentration,
maximum bud weight was obtained when the solution was un- irradiated. At 1000ppm concentration, maximum average bud weight was obtained when the solution was irradiated at 40 kGy radiation dose.
Table 02: Effects of irradiated chitosan on average leaf area and average weight per bud of tea
Radiation Dose | Average Leaf Area(cm2) | Average Weight per Bud(g) | ||||
Radiation Dose | Concentration | Concentration | ||||
Radiation Dose | 300 ppm | 500 ppm | 100ppm | 300 ppm | 500 ppm | 1000 ppm |
0 kGy | 14.89±1.02 | 12.18±0.98 | 13.11±0.87 | 0.368±0.025 | 0.329±0.023 | 0.395±0.032 |
20 kGy | 15.14±1.09 | 13.01±1.13 | 13.39±1.07 | 0.309±0.024 | 0.360±0.031 | 0.308±0.022 |
30 kGy | 16.13±1.11 | 20.17±1.80 | 14.69±0.93 | 0.335±0.028 | 0.381±0.031 | 0.347±0.027 |
35 kGy | 15.76±0.89 | 16.70±1.42 | 15.45±1.39 | 0.335±0.023 | 0.378±0.029 | 0.339±0.025 |
40 kGy | 15.06±1.25 | 21.25±1.47 | 19.59±1.42 | 0.302±0.020 | 0.411±0.031 | 0.430±0.030 |
50 kGy | 14.72±0.98 | 19.85±1.38 | 16.24±1.03 | 0.345±0.029 | 0.437±0.037 | 0.367±0.031 |
Control | 15.48±1.78 | 0.393±0.032 |
Fungus was counted in various petri plates after incubation. Total numbers of fungi were calculated by colony counter in PDA
media.From figure 05, fungal count for1000 ppm chitosan solution at different radiation doses can be ranked as follows:
Control > Un-irradiated > 20 kGy > 30 kGy > 35 kGy > 40 kGy > 50 kGy
For control, the total fugal count was 302.4×102, which is reduced to
6.2×102 when plants were treated with un-irradiated chitosan at a concentration of 1000 ppm. It was found that, the anti-fungal activity of chitosan is increased with the increasing rate of radiation doses. If we
consider 1000 ppm concentrated chitosan treatment, we found that, with the increasing rate of radiation doses, total fungal count was decreased.
40000
30000
30240
20000
10000
0
6200 4920 3680
1360 280 244
Control Un-irradiated 20 kGy 30 kGy 35 kGy 40 kGy 50 kGy
Treatment
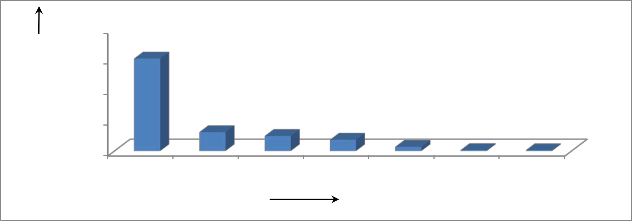
Figure 05: Anti-fungal activity of radiation processed chitosan on tea plants
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1697
ISSN 2229-5518
It has been demonstrated that, several exogenous and endogenous factors regulate the growth, development and yield of a plant [15]. Among exogenous factors, various plant growth promoters are known which have direct or indirect influence on growth of the plant. From this experiment it was found that, viscosity of chitosan solution changed with radiation doses. It had been reported that, the main factors affecting the antibacterial activity of chitosan are molecular weight (MW ) and concentration [16]. The molecular weights of polysaccharides are directly related to its viscosity [17]. It has already been reported that polysaccharides such as chitosan, alginate, and carrageenan in their depolymerised form have the novel properties of promotion of germination and shoot elongation [5, 18, 19].
Oligomers, produced by depolymerization of chitosan, have been
reported to have triggered the stimulation of growth, promotion of germination and shoot elongation in plants. Among the different concentrations of radiation degraded chitosan (irradiated at 40 kGy),
500 ppm concentration exposed promotive effects on all the growth parameters studied [20]. In lines with the present results, it is earlier reported that significant improvement in plant growth attributes by the application of radiation-derived chitosan. Also, Hien et al. reported that when the range of 10 to 500 kGy Co-60 gamma rays exposed chitosan, proved very effective for considerable plant growth promotion [11]. Furthermore, the reports also show that, chitosan when irradiated at suitable radiation dose, and applied on plants through hydroponics system or through foliar application, has become a successful method in modern commercial farming. The results are in conformity with the findings of several researchers in case of various crops [4, 19, 21, 22,
23, 24].
Since, the important role of oligosaccharides (degraded chitosan) in inducing cell signaling in various plants leading to stimulation of various physiological processes had been revealed [20], the application of irradiated chitosan in this study increased the average leaf area and number of buds. Furthermore, the absorption of oligomers acted as a growth promoter, which resulted in plant root and shoot elongation and, thereby led to promote and increase plant productivity and improvement in physiological parameters compared with the unsprayed plant [25]. Due to this property, the plants treated with radiation degraded alginate show an increase not only in growth but also in disease resistant capacity. In the present study, we noticed that activities of various fungi were positively regulated by various concentrations of chitosan at different radiation doses. In this regard, our findings are similar to those that report the synthesis of certain enzymes in the tissue culture after addition of polysaccharides derived oligomers [24].
Luan et. al. had found that, the molecular weight of chitosan was decreased with the increase in the radiation dose [21]. They also found
that, low molecular weight of chitosan had progressive impact on plant growth. From this study it was also found that, growth promoting activity of chitosan is increased with higher radiation doses i.e. at low molecular weight, but at very high radiation doses the growth promoting activity of irradiated chitosan was decreased. Previous studies have shown that a range of concentrations of radiation degraded polysaccharides depend upon the source and unit (kGy) of irradiation for a particular plant [26]. Provably this may be a cause for that; in this study there were some variations in contrast with other species of plants.
From present study it was found that, chitosan was capable to reduce total fungal count in tea plants. The total fungal count was reduced with
the increasing rate of radiation doses at 1000 ppm than control which has similarity with the work of Yang et. al. [27].
The study had an opportunity to look into potential uses of biomaterials as they are cheap, eco-friendly, non-harmful, and available in nature. Based on the present study, it can be concluded that, radiation processed biomaterials has progressive impact on tea plants in terms
*Corresponding Author: M. A. Khan
Email: makhan.inst@gmail.com
of productivity and anti-fungal activity. Radiation degradation of natural biomaterials has important effects on viscosity, which is also related to its molecular weight. Tea plant showed different responses to biomaterials at various radiation doses and concentrations. From this study, it was found that, based on fresh weight of buds, chitosan solution at 500 ppm concentration with 40 kGy radiation doses were found to increase the productivity of tea plants at a rate of about 38% and bud weight were found to increase at a rate of 32% at the same dose. Besides this, 1000 ppm concentrated chitosan solution radiated at 40 kGy was proved to reduce fungal count more than 100 times in contrast with the control. Further experiment will be related to the study of growth promoting and antifungal activity of biomaterials throughout the year as well as their impact on plucking round, bio-chemical composition and quality of made tea.
Authors are highly acknowledged to Ali Baher Tea State, Sylhet, Bangladesh, for their cordial cooperation during the research work.
[1] M. Z. I. Mollah, M. A. Khan, R. A. Khan, “Effect of gamma irradiated sodium alginate on red amaranth (Amaranthus cruentus L.) as growth promoter”, Radiation Physics and Chemistry, vol. 78, pp. 61-64, 2009.
[2] K. Ohta, A. Zaniguhi, N. Konishi, T. Idosoki, “Chitosan treatment affects plants growth and flower quality in Eustoma grandiflorum”, Horticulture Science, vol. 34, pp.
233-234, 1999.
[3] C. Akimoto, H. Aoyagi, and H. Tanaka, “Endogenous elicitor-
like effect of alginate on physiological activities of plant
cells”, Applied Microbiology and Biotechnology, vol. 52, pp.
429–436, 1999.
[4] M. E. Haque, R. A. Khan, N. C. Dafader, and M. Z. I. Mollah,
, Country Report of Bangladesh on the Activities on“Modification of Natural Polymers by Radiation Processing for Value Added Products”, pp.46-53, 2009.
[5] A. Darvill et. el., “Oligosaccharins-oligosaccharides that regulate growth, development and defense response in plants”, Glycobiology, vol. 2, pp.181-198, 1992.
[6] M. Hafez, O. Muhamad, S. Y. Muhammad, M. S. Ahmad, “Irradiated chitosan as plant promoter”, Prosiding Simposium Biologic Gunaan Ke-7, Mines Beach Resort and Spa, Seri Kumbangan, Selangor, Malaysia, 2003.
[7] S. M. Hudson, C. Smith, “Polysaccharide: Chitin and Chitosan: Chemistry and technology of their use as structural materials”, In Biopolymers from Renewable Resources, Springer-Verlag, New York, USA, pp.96, 1998.
[8] C. E. Kast, W . Frick, U. Losert, and A. Bernkop-Schnürch, “Functional properties of chitin and chitosan”, International Journal of Pharmaceutics, pp.256, 183, 2003.
[9] A. El Ghaouth, J. Arul, J. Grenier, and A. Asselin, “Effects of Chitosan on Cucumber Plants: Supperession of Pythium aphanidennatum and induction of defence reactions”, Phytopathology, vol. 84, pp. 313-320, 1992.
[10] L. A. Hadwiger, “Chitosan: natural regulator in plantfungal pathogen interaction increases crop yields”, Academic Press: San Diego, pp. 4, 1984.
[11] N. Q. Hien et. el., “Growth promotion of plants with depolymerised alginates by irradiation”, Radiation Physics and Chemistry, vol. 59, pp. 97- 101, 2000.
[12] N. Q. Hien, “Radiation degradation of chitosan and some biological effects”, Radiation Processing of Polysaccharides, IAEA-TECDOC-1422, pp. 67-73, 2004.
[13] T. Freier, “Processing of Chitosan and Chitosan
Derivatives”, www.faqs.org/patents/app/20080254125, 2008
.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1698
ISSN 2229-5518
[14] A. O. A. C., “Official Methods of Analysis”, Association of
Official Analytical Chemists, 17th Ed., Gaithersburg,
[15] N. K. Srivastava, and A. K. Srivastava, “Influence of
Maryland. U.S.A., 2000.
[22] L. X. Thama et. el., “Effect of radiation-degraded chitosan on
gibberellic acid on 14CO
metabolism, growth, and
plants stressed with vanadium”, Radiation Physics and
production of alkaloids in Catharanthus roseus”,
Photosynthetica, vol. 45, pp. 156-160, 2007.
[16] N. Liu et. el., “Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli”, Carbohydrate Polymers, vol. 64, pp. 60–65, 2006.
[17] R. H. Chen, and H. D. Hwa, “Effect of MW of chitosan with same degree of deacetylation on the thermal, mechanical, & permeability properties of the prepared membrane”, Carbohydrate Polymers , vol. 29, vol. 335–358, 1996.
[18] L. Q. Luan et. el., “Biological effect of radiation-degraded alginate on flower plants in tissue culture”, Biotechnology and Applied Biochemistry, vol. 38, pp. 283–288, 2003.
[19] T. Kume, N. Nagasawa, F. Yoshii, “Utilization of carbohydrates by radiation processing”, Radiation Physics and Chemistry, vol. 63, pp. 625-627, 2002.
[20] N. Q. Hien, L. Q. Luan, T. T. Hanh, and L. Hai, “Radiation Processing Technology for Production of Plant Growth Promoter From Brown Seaweed and Plant Protector from Shrimp Shell”, Nuclear Research Institute, Dalat, Vietnam. IAEA-SM-365/23, pp.45, 2009.
[21] L. Q. Luan et. el., “Characterization and Biological Effect of Radiation Degraded Chitosa”,. Proceedings of International Workshop on Biotechnology in Agriculture, pp.23-28, 2006.
Chemistry, vol. 61, pp. 171-175, 2001.
[23] S. Albayrak, and N. Camas, “Effects of different levels and application times of humic acid on root and leaf yield and yield components of forage turnip (Brassica rapa L.)”, Journal of Agronomy, vol. 4, no. 2, pp. 130-133, 2005.
[24] E. E. Farmer, D. M. Thomas, J. S. Michael, and A. R.
Clarence, “Oligosaccharide signaling in plants”, Journal of
Biological Chemistry, vol. 266, pp. 3140-3145, 1991.
[25] El-Rehim et. el., “Radiation Modification of Natural
Polysaccharides for Application in Agriculture”, Report of the
2nd RCM on "Development of radiation-processed products of natural polymers for application in agriculture, healthcare, industry and environment", IAEA, Viena, pp.85-94, 2009.
[26] Y. Tomoda, K. Umemura, and T. Adachi, “Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate (A.L.L.)”, Bioscience, Biotechnology and Biochemistry, vol. 58, pp. 203-213, 1994.
[27] M. Y. Yang, H. T. Lin, T. H. W u, and C. C. Chen, “W ettability and Antibacterial Assessment of Chitosan Containing Radiation-Induced Graft Nonwoven Fabric of Polypropylene- g-Acrylic Acid”, Journal of Applied Polymer Science, vol. 90, no. 5, pp. 1331-1336, 2003.
IJSER © 2013 http://www.ijser.org