International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1401
ISSN 2229-5518
Fluorescent Bacillus endophyticus AVP9-Multiple potential for phosphate solubilization, plant growth promotion and bio control†
NOKKU PRADEEP KUMAR AND AMRUTHA V AUDIPUDI*
![]()
The term Rhizobacteria is used to describe a subset of rhizosphere bacteria capable of colonizing the root environment [1] [2].Beneficial, root colonizing, and plant growth promoting (PGP) rhizobacteria, are defined by three intrinsic characteristics: (1) must be able to colonize the root (2) must survive and multiply in microhabitats associated with the root surface, in competition with other micro biota, and (3) must promote plant growth![]()
• Pradeep kumar Nokku Research scholar Research area: PGPR
Department of Microbiology
Acharya Nagarjuna University, Guntur,A.P.India phosphatesolubiliser@gmail.com
• Dr.A.Amruthavalli:
Assistant Professor,
Department of Microbiology
Acharya Nagarjuna University,
Guntur,A.P,India. audipudi_amrita@yahoo.com
Plant growth promoting rhizobacteria when applied to seeds/soil or crops, enhance the growth of the plant directly by providing nutrients to plants or indirectly by reducing the damage from soil- borne plant pathogens [3].The Concept of rhizosphere was a narrow zone of soil surrounding the roots where microbe populations are
stimulated by root activities [4] has now been extended to include the soil surrounding a root in which physical, chemical and biological properties have been changed by root growth and activity [5]. Since bacteria are the most abundant microorganisms in the rhizosphere, it is highly probable that they influence the plants physiology to a greater extent, especially considering their competitiveness in root colonization [6] [7] [8]. Plants select those bacteria contributing most to their fitness by releasing organic compounds through exudates [9] creating a very selective environment where diversity is low [10] .Rhizobacteria inhabit plant roots and exert a positive effect ranging from direct influence mechanisms to an indirect effect. So, the bacteria inhabiting the rhizosphere and beneficial to plants are termed PGPR [3]. In the last few years, the number of PGPR that have been identified has seen a great increase, mainly because the role of the rhizosphere as an ecosystem gained importance in the functioning of the biosphere. Various species of bacteria like Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Bacillus and Serratia have been reported to enhance the plant growth [11] [12].There are several PGPR inoculants currently commercialized that seem to promote growth through at least one mechanism; suppression of plant disease (Biocontrol), improved nutrient acquisition (Bio fertilizers), or phytohormone production (Bio stimulants), induction of systemic resistance, and production of siderophore or antibiotics. Exposure to the PGPR triggers a defense response by the crop as if attacked by pathogenic organisms. Siderophore produced by some PGPR scavenge heavy metal micronutrients in the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1402
ISSN 2229-5518
rhizosphere (e.g. iron) starving pathogenic organisms of proper nutrition to mount an attack of the crop. Antibiotic producing PGPR releases compounds that prevent the growth of the pathogens.
Phosphates and other nutrient are also solubilized by PGPR strains to increase the availability of Phosphorus for plants in soil with large amount of precipitated phosphates [13] and nitrogen fixation. These bacteria are also capable to suppress the growth of deleterious microorganisms by production of siderophore, β 1, 3 glucanases, chitinases and antibiotics [14] .Siderophore producing bacteria promote plant growth indirectly by sequestrating the limited iron in the rhizosphere and reduce availability for growth of phytopathogens [15] several fluorescent Pseudomonas and Bacilli have been used as seed or root inoculants for higher growth yield of various crops [11]. The common traits of growth promotion includes production or changes in the concentration of plant hormones such as Auxin, gibberellins, cytokinins and ethylene. Indole acetic acid (IAA) is one of the most physiologically active auxin. IAA is release as secondary metabolite because of rich supplies of substrates exuded from the roots [16][17].Microbial biosynthesis of IAA in soil is enhanced by tryptophan secreted from roots or decaying cells [18].Gibberellins are implicated in promotion of root growth, root hair abundance and inhibition of floral bud differentiation in woody angiosperms, regulation of vegetative and reproductive bud dormancy and delay of senescence in many organs of a range of plant species [19][20][21].
Bacillus is the most abundant genus in the rhizosphere, and the PGPR activity of some of these strains has been known for many years, resulting in a broad knowledge of the mechanisms involved [22][23]. There are a number of metabolites that are released by these strains [24] which strongly affect the environment by increasing nutrient availability of the plants [25]. Bacillus is also found to have potential to increase the yield, growth and nutrition of raspberry plant under organic growing conditions [26].Naturally present in the immediate vicinity of plant roots, B. subtilis is able to maintain stable contact with higher plants and promote their growth. Bacillus licheniformis when inoculated on tomato and pepper shows considerable colonization and can be used as a Biofertilizer without altering normal management in greenhouses [27]. Jaizme-Vega et al., 2004 evaluated the effect of a rhizobacteria consortium of Bacillus spp. on the first developmental stages of two micro propagated bananas and concluded that this bacterial consortium can be described as a prospective way to increase plant health and survival rates in commercial nurseries. Bacillus megaterium is very consistent in improving different root parameters (rooting performance, root length and dry matter content of root) in mint[28] .The PSB Bacillus megaterium var.
phosphaticum and Potassium Solubilizing Bacteria (KSB) Bacillus mucilaginous when inoculated in nutrient limited soil showed that rock materials (P and K rocks) and both bacterial strains consistently increased mineral availability, uptake and plant growth of pepper and cucumber, suggesting its potential use as fertilizer[29,30] .The Bacillus pumilus 8N-4 can be used as a bio-inoculant for bio fertilizer production to increase the crop yield of wheat variety Orkhon in Mongolia [31].In the present investigation multiple potential of rhizobacteria isolated from chilli rhizosphere has been characterized in terms of phosphate solubilization, PGP traits, biocontrol and Acid phosphatase.
Bacteria exhibiting orange fluorescence (AVP 9) were isolated from chilli rhizosphere peddakurapadu, Guntur district of Andra Pradesh in India, on normal nutrient agar medium with Glucose at pH7.0, Temperature 370c with incubation period 48 hrs. The bacterial isolate was characterized by its cultural conditions, morphological and biochemical characteristics [32].
The isolate was screened for phosphate solubilization as described by Gupta S. et al., 1994. On modified Pikovskaya agar with insoluble Tricalcium phosphate (TCP). A loop full of culture was placed on the center of agar plate and incubated at 30±0.1 °C for 5 days. The Solubilization zone was determined by subtracting the diameter of bacterial colony from the diameter of total zone.
Inorganic phosphate Solubilization was quantitatively estimated [33][34]. Bacterial isolate was grown in National Botanical Research Institute’s Phosphate (NBRIP) broth containing 0.5% Tricalcium phosphate (TCP).500µl of bacterial inoculum was added to 50 ml of medium and incubated at 30±0.1 °C at 180 rpm for 5 days in Incubator Shaker and uninoculated medium was taken as control. The culture was centrifuged at 10,000 rpm for 10 min. Inorganic phosphate present in supernatant was estimated by vanado-molybdate-yellow color method by using Barton’s reagent and 0.5 ml of the supernatant was added to 2.5 ml Barton's reagent and volume was made to 50 ml with de- ionized water. After 10 min of incubation the absorbance was read at 430 nm in UV/Visible Spectrophotometer and the total soluble phosphorous was calculated from the regression equation of standard curve. And values were expressed in ppm. The pH of culture supernatant was also measured. The experiment was repeated in triplicates.
Growth optimization of isolate was studied at different temperatures (25ºc,37 ºc,50 ºc), pH ranging
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1403
ISSN 2229-5518
from(3,5,7,9,12),NaCl(0.3%,0.5%,0.7%,0.9%,1%),Carbon sources(Sucrose ,Maltose, Lactose, Dextrose)
IAA production was detected [35].Quantitative analysis of IAA was performed [36] at a concentration of 1000µg/ml of tryptophan. Bacterial culture grown for 48hrs on the respective media at 30±1°C.Fully grown culture was centrifuged at 10,000rpm for 10 minutes. The supernatant (1 ml) was mixed with 4 ml of Salkowski reagent (50ml, 35% of perchloric acid,1 ml 0.5M FeCl3 solution) with few drops of Orthophosphoric acid. Development of pink colour indicates IAA production. Optical density was taken at
535nm in UV/Visible Spectrophotometer. Concentration of IAA produced by culture was measured with the help of standard graph f IAA obtained in the range of 10-100 µg
/ml.
Ammonia production was estimated by Nesslerization reaction. Freshly grown culture was inoculated into 4ml of peptone water and incubated for 48hrs at 370c.Broth was collected, centrifuged and 1ml Nessler’s reagent was added to 1ml of supernatant and the volume of this mixture was made up to 10ml by addition of ammonia free distilled water. Development of brown to yellow color was a positive test for ammonia production and optical density was measured by spectrophotometer at 450nm [37] .The concentration of ammonia was estimated based on a standard curve of ammonium sulfate ranging from 0.1 to
1µmol ml-1.
Acid phosphatase activity was also estimated [38]. Cells grown overnight in citrate salt medium(g/l)[trisodiumcitrate,3;K2 HPO4, 10.5;KH2 PO4 ,5.4;( NH4 )2 SO4, 1.2;MgSO4,0.4;CaCl2, 0.15 (P H7.0)] were harvested by centrifugation at 8,000 rpm for 8 min.Cell pellets were suspended in normal saline (O.D of 1.0 at 600 nm) .Incubation mixture for acid phosphatase enzyme assay contained 50 µl of cell suspension ,50 µl of 0.12 M pNPP ( p-nitrophenyl phosphate) and 500µl of 50mMTris- Malate buffer (pH5.3).The suspended cells were incubated for 30 min at 30˚c.After centrifugation ,1 volume of 0.5 M NaOH was added to 1 volume of supernatant ,and O.D420 was measured .Results were expressed in µmol units of product formed per OD600 of cells .One unit was defined as the hydrolysis of 1µM of pNPP to pNP( p-nitrophenol) per minute at 30˚c.
Presence of catalase was checked qualitatively [39] .Six percent hydrogen peroxide was added on the colonies grown on nutrient agar plates; effervescences of O2 released from the bacterial colonies indicate the presence of catalase activity.
Qualitative estimation of HCN production was done by Picrate assay[40] .Nutrient agar medium was amended with 4.4g glycine L-1 and bacterium was streaked on plate. A whatman filter paper no.1 soaked in 2% sodium carbonate in 0.5% picric acid solution was placed between the base and the lid of the petri dish. Plates were sealed with Para film and incubated at 27±20c for 4 days. After incubation, the color change of filter indicates the release of cyanide from bacterial isolate.
Pure culture of bacterial isolate AVP 9 was grown until log phase was attained and genomic DNA was isolated essentially according to Bazzicalupo [41]. The amplification of 16S rRNA gene was done by using universal bacterial primer 1492R (5´-TACGGYTACCTTGTTACGACTT-3´) and
27F (5´ AGAGTTTGATCMTGGCTC AG- 3´) as per the conditions described by Pandey [42]. The PCR product was sequenced at Indian Institute of Horticulture Research, Hasserghat, and Bangalore. The sequences obtained were compared with those from the GenBank using the BLAST program [43] and Phylogenetic trees reconstructions were obtained by the Neighbor joining method 1000 bootstrap replicates were performed to assess the statistical support for each branch in the tree[43,44]
Antagonism against phytopathogen was determined by agar well diffusion method[45] The molten nutrient agar was inoculated with Xanthomonas campestris (MTCC NO) supplied by MTCC chandighar, a phytopathogen and poured into the Petri plate, 100µl of AVP9 was inoculated into the well. The plates were incubated overnight at 37˚c temperature and antagonism was determined by measuring the diameter of zone of inhibition.
Out of 55 bacteria isolated from chilli rhizosphere, one of the colonies AVP9 exhibited orange fluorescence under UV light of longer wave length on the basis of culture, morphological, biochemical and molecular characteristics; the bacterial isolate was identified as Bacillus endophyticus AVP 9. (Table1)
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1404
ISSN 2229-5518
Morphological and physiological characteristics | |
Test | AVP9 |
Morphology Arrangement | Rod& single |
Gramstaining/ Pigmentation | Purple& Orange flourescent |
Motile | +ve |
Urease | -ve |
Starch | +ve |
Glucose | -ve |
Lactose | -ve |
Sucrose | -ve |
Biochemical characteristics |
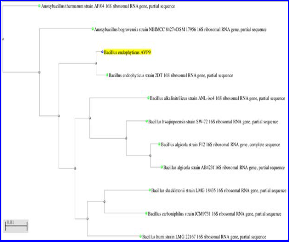
1000bp PCR product of 16SrRNA gene was amplified from genomic DNA of Bacillus endophyticus AVP 9 strain.16S ribosomal RNA partial gene analysis was done at Macrogen South Korea. Phylogenetic analysis of 1000bp of fasta sequence by BLAST, NCBI revealed that the strain AVP 9 showed 99% similarity with Bacillus endophyticus
2DT.Hence the sequence was submitted in genbank NCBI
with a name Bacillus endophyticus AVP 9 Figure 1. (Accession No. KF 527823).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1405
ISSN 2229-5518


A.Phosphate solubilization * | B. Antagonism ** |
*Phosphate solubilization by showing zone of clearance.
** AVP9 showing zone of inhibition against
X.campestris
characterization of AVP 9 isolate of phosphate solubilization showed zone of clearance on Pikovskaya’s agar medium after 4 days .(Plate-1A)
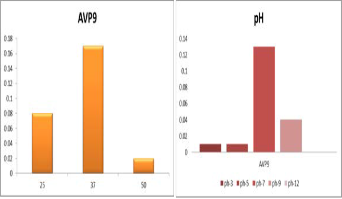
AVP 9 showed variation in growth in different carbon sources such as Sucrose, Maltose, Lactose and Dextrose (Figure 2a). Maximum growth was observed in Lactose and found to be very minimum in Dextrose. It is observed that growth pattern of AVP -9 showed variation at different concentrations of NaCl. AVP 9 showed maximum growth
rate at 0.7% NaCl and moderately high growth rate at 0.9% NaCl (Figure 2b). It is also observed that AVP 9 showed growth variation at different temperatures and found to be maximum at 37˚c and moderate at 25˚c.(Figure 2c)
.Growth at different pH ranging from pH3.0- pH 12 also
showed variation and observed to be high at pH7 and
relatively moderate at pH9 (Figure 2d) Based on the results cited in fig1, growth of AVP- 9 was optimized in a medium containing Lactose, 0.7%NaCl,pH7 at 37˚c temperature.
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1406
ISSN 2229-5518
a.Effect of Carbon sources | b. Effect of NaCl |
|
|
c. Effect of Temperature | d. Effect of Ph |
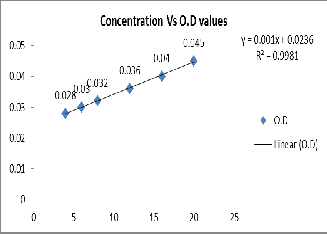
Results revealed that the solubilization of tricalcium phosphate was progressively increased for 7 days and gradually declined in 10th day and 13th day. .Phosphate Figure 3.
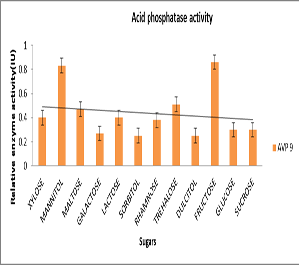
Results revealed that AVP 9 showed high acid phosphatase activity (0.205 IU/ml) at pH3.4 (Figure 4). For the first time, acid phosphatase activity of AVP 9 was also estimated quantitatively at 12 different sugars and 15 amino acids and relative activity was measured in international units (IU). It
solubilization was observed to be maximum (1112ppm) on
7th day and also noted that the solubilization was gradually
decreased with rapid decline of pH from 7 to 4(Figure 3).
has been observed that the isolate showed acid phosphatase activity in all 12 different types of sugars. Figure 5 revealed that the activity was very high in Fructose (0.86 IU/ml), Mannitol (0.83 IU/ml) and Trehalose (0.51IU/ml). (Figure
5). Growth and acid phosphatase production of AVP9 was
studied at 12 different sugars. It was observed that
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1407
ISSN 2229-5518
Mannitol and Fructose proportionately enhances the enzyme activity along with growth. Enzyme activity was not proportionately increased along with growth in presence of Trehalose. It clearly indicates that sugars like Fructose and Mannitol act as inducers for enzyme acid phosphatase. Effect of sugars on growth of AVP9 was also studied (Figure 6) and observed that the growth is very Figure 4.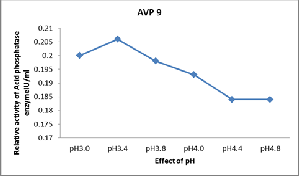
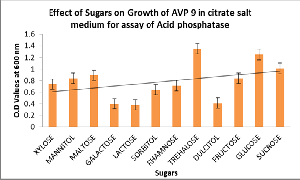
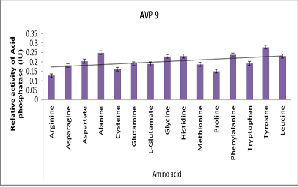
high in sugars like Xylose, Mannitol, Maltose, Trehalose, Glucose and sucrose. and also found that the growth was maximum in Trehalose. AVP 9 isolate also showed acid phosphatase activity in all 15 different types of amino acids. No significant increase of acid phosphatase activity was observed (Figure 7).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1408
ISSN 2229-5518
Plant growth promoting traits such as IAA production, ammonia production, siderophore production, HCN production and antagonism against phytopathogen was studied in AVP9 under optimized growth conditions (Table
2and Plate-1B). IAA and ammonia production were
quantitatively estimated and observed that AVP9 produced
80µg/ml of IAA and 110µg/ml of ammonia .Siderophore
and HCN production was qualitatively estimated and observed that AVP9 was positive to siderophore production and HCN production.AVP9 showed antagonism against Xanthomonas campestris.
IAA(µg/ml) production | 80 |
Ammonia(µg/ml) Production | 110 |
Siderophore Production | Positive |
HCN Production | Positive |
Catalase production | Positive |
Antagonism against X. campestris | Positive |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1409
ISSN 2229-5518
Nitrogen and phosphorus are two most limiting nutrients in the soil as well as plant enhancing nutrients[46].Phosphate fertilizer represents a high cost to the former and most of the soils are poor in available phosphorus contents and therefore it is of interest to take advantage of soil microorganisms for the mobilization of phosphorus in the soil [47]. In present investigation orange fluorescent Bacillus AVP9 was screened invitro for phosphate solubilization and Acid phosphatase activity both qualitatively and quantitatively. After seven days of incubation the isolate AVP9 showed high phosphate solubilization. 37˚c Temperature, 0.7%, NaCl (salinity), pH
7 and Lactose were identified as influencing factors for
optimization of growth and maximum phosphate solubilization. In the present study AVP9 showed significant production of ammonia and strong phosphate solubilization. This infers that AVP9 isolate in the rhizosphere makes ammonia and phosphorus available to the plant by which nutritional needs of the plant can be fulfilled.
Certain strains of fluorescent Pseudomonas as
PGPR as they promote plant growth by secreting auxin, gibberellins and cytokinins[48]. Fluorescent pseudomonads are non- pathogenic rhizobacteria [49] and several isolates of P.fluorescence, P.putida P.aeruginosa, and P.aureofaciens suppressed the soil borne pathogens through different proposed mechanisms including rhizosphere colonization,antibiosis and iron chelation by siderophore production [49].AVP9 isolate showed multiple plant growth promoting activities similar to the findings of isolates of India which are commonly explore. Bacillus endophyticus exhibiting yellow fluorescence isolated from cotton rhizosphere extensively reviewed for its antibiotic metabolites [50] .First time we are reporting an orange fluorescent Bacillus AVP9 for its multiple potential in plant growth promotion as well as extracellular phosphate solubilization and acid phosphatase activities. Previous reports revealed isolates Azotobacter, Pseudomonas, Bacillus showed multiple plant growth promoting traits which may promote plant growth directly/indirectly/synergistically. Production of siderophore is another important trait of rhizosphere bacteria bind to the available form of Fe++, thus making non-available to phytopathogens. In this way this trait indirectly influences plant growth It has been reported that isolate AVP9 can produce 80ug/ml of IAA, positive for REFERENCES
1. KLOEPPER,JW,R.M.ZABLOTOWICS, E.M. TIPPING and R.LIFSHITZ. Plant growth promotion mediated by Bacterial Rhizosphere Colonizers. In: The Rhizosphere and Plant Growth, Keister,D.L.ad P.B.Cregan(Eds.)Kluwer Academic Publishers,USA,315-326, 1991.
HCN and siderophore production similar to the findings of Haas and Keel et al: Gupta etal 1998. Dual culture of AVP9 with Xanthomonas campestris also proved that isolate AVP9 is a potential antagonist against phytopathogenic bacteria as a marker of biocontrol agent for protecting the plant from biotic stresses. This in turn can indirectly enhance the plant growth [51] .Prevoius studies revealed the production of siderophore and HCN and other secondary metabolites of Pseudomonas strains were most effective in control of plant pathogens[52].Similarly in present study antibacterial activity of AVP9 assumed to be synergistic effect of siderophore production and HCN production against X
.campestris. This shows that multiple potential of AVP9 can help in plant protection and enhance plant growth.
In this study we also made an attempt to characterize acid phosphatase enzyme activity at different pH, different carbon sources and amino acids. Best of our knowledge, relation between growth of isolate and acid phosphatase activity has not been reported so far. First time an attempt was made to correlate growth of isolate with acid phosphatase activity at different carbon sources. Our reports revealed that there is no direct correlation between bacterial growth and enzyme activity in presence of carbon sources. However some of the carbon sources such as Mannitol and Fructose act as inducers and enhance the activity of acid phosphatase. No significant enhancement of acid phosphatase activity was observed in presence of amino acids. To hornets the dual potential of AVP9 in the field of Agriculture and Industry, future studies should be carried out.
A novel bacterial strain namely: Bacillus endophyticus AVP 9 was isolated from Peddakurapadu chilli rhizosphere soil sample, Guntur,A.P. The fact that Bacillus endophyticus AVP
9 to be positive for various PGPR characteristics suggests
that the isolate have better potential for green house and field testing and application improving yield of chilli.
I am thankful to UGC SPECIAL ASSISTANCE PROGRAMME BASIC SCIENCE RESEARCH (UGC SAP- BSR) New Delhi for financial assistance to N.Pradeep Kumar as UGC SAP-BSR fellow, From Department of Bota ny and microbiology ANU, Guntur.
2. KLOEPPER, JW. Plant growth promoting Rhizobacteria(Other systems).In: Azospirillum/Plant Associations Okon,Y.(Eds.).CRC Press,Boca Raton,FL,USA,111-118, 1994.
3. KLOEPPER, JW, LEONG, J,TEINTZE, M,
SCHROTH,MN, Enhanced plant growth by
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1410
ISSN 2229-5518
siderophores produced by plant growthpromoting rhizobacteria. Nature, 286: 885–886, 1980
4. HILTNER L. Uber neuere erfahrungen und probleme auf dem gebiet der boden bakteriologie und unter besonderer berucksichtigung det grundungung und branche. Arb. Deut. Landw. Ges, 98: 59-78, 1904
5. MC CULLY M. The rhizosphere: the key functional
unit in plant/soil/microbial interactions in the field. Implications for the understanding of allelopathic effects. In Proceedings of the 4th World Congress on Allelopathy: Charles Sturt University, Wagga Wagga, NSW, Australia. International Allelopathy Society. Edited by Harper J, An M, Wu H, Kent J, 21-26 August
2005
6. ANTOUN H, KLOEPPER, JW, Plant growth promoting rhizobacteria (PGPR). In Encyclopedia of Genetics. Academic Press, New York. Edited by Brenner S, Miller JH, 1477–1480, 2001.
7. KLOEPPER, J.W., SHAW, J.J and CHALIFOUR,F.-P.
Root colonization of faba bean (Vicia faba L.) and pea (Pisum sativum L.) by Rhizobium leguminorarum bv. viciae in the presence of nitrate nitrogen,Can. J. Microbiol, 47:1068-1074, 2001.
8. BARRIUSO, J, SOLANO, BR, LUCAS, JA, LOBO, AP,
VILLARACO, AG, MAÑERO, FJG, Ecology, Genetic Diversity and Screening Strategies of Plant Growth Promoting Rhizobacteria (PGPR). WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Edited by Ahmad I, Pichtel J, Hayat S, 1-17, 2008.
9. LYNCH, JM, The Rhizosphere. John Wiley & Sons Ltd, Chichester, Edited by Lynch JM, 458, 1990.
10. GARCÍA, JL., PROBANZA, A., RAMOS, B., MAÑERO,
FJG, Ecology, genetic diversity and screening strategies of plant growth promoting rhizobacteria. Journal of Plant Nutrition and Soil Sciences, 164: 1–7, 2001.
11. GLICK, BR, The enhancement of plant growth by free
living bacteria. Canadian Journal of Microbiology, 41
(Suppl 2): 109–114, 1995.
12. JOSEPH, B., PATRA,RR.,
LAWRENCE,R,Characterization of plant growth promoting Rhizobacteria associated with chickpea (Cicer arietinum L). International Journal of Plant Production, 1 (Suppl 2): 141-152, 2007.
13. GOLDSTEIN,A.H, Bacterial solubilization of mineral
phosphate: Historic perspective and future prospects.Am.J.Altern.Agric,1: 51-57, 1986.
14. CATTELAN,A.J.,HARTEL,P.G.,FUHRMANN,J.J,
Screening for plant growth promoting Rhizobacteria to promote Early Soybean Growth,Soil Sce Soc Am J
63:1670-1680, 1999.
15. ALEXANDER, B.D., ZEEBERI, D.A, Use of
chromazurol S to evaluate siderophore production by
rhizosphere bacteria .Biol.Fertil.Soils, 2:39-54, 1991.
16. AHMED,F.,AHMAD,I.,KHAN,M.S, Indole acetic acid production by the indigenous isolated of Azotobacter and Fluorescent Pseudomonas in the presence and absence of tryptophan, Turk.J.Biol,29:29-34, 2005.
17. SRRZELCZYK,E.,POKOJSKABURDZEIJ,A, Production of auxin and gibberellin like substances by mycorrhizal fungi,bacteria and actinomycetes isolated from soil and mycorrhizosphere of pine(Pinus silvestris L.)Plant and Soil.81:185-194, 1984
18. BENEZRI,E,COURTADE,A,PICARD,C.&GUCKERT,A, Role of maize root exudates in the production of auxins by Pseudomonas fluorescence M.3.1.Soil Biol and Biochem
30:1481-1484, 1998.
19. BOTTINI, R., LUNA, V, 1993. Bud dormancy in
deciduous fruit trees. Curr Top Plant Physiol 1:147-159.
20. FULCHIERI, M., LUCANGELI, C., BOTTINI,R,
Inoculation with Azospirillum lipoferum affects growth
and gibberellin status of corn seedling roots, Plant Cell
Physiol 34: 1305-1309, 1993.
21. RIENOSO, H., DAURIA, C., LUNA, V.,PHARIS,R&BOTTINI ,R, Dormancy in peach (Prunus persica L.) flower buds VI.Effects of gibberellins and an acylcyclohexanedione (Cimectacarb) on bud morphogenesis in field experiments with orchard trees and on cuttings .Can J Bot 80,656-663, 2002.
22. PROBANZA, A., LUCAS GARCÍA, JA., RUIZ
PALOMINO, M., RAMOS, B., GUTIÉRREZ MAÑERO,
F.J, Pinus pinea L seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus (B. licheniformis CECT 5106 and B. pumilus CECT 5105). Applied Soil Ecology, 20 (Suppl 2): 75–84,
2002.
23. GUTIÉRREZ MAÑERO, FJ, PROBANZA, A, RAMOS, B, COLÓN FLORES, JJ, LUCAS GARCÍA, JA, Ecology, Genetic Diversity and Screening Strategies of Plant Growth Promoting Rhizobacteria (PGPR). Journal of Plant Nutrition, 26 (Suppl 5): 1101–1115, 2003.
24. CHAREST, MH, BEAUCHAMP, CJ, ANTOUN H, Effects of the humic substances of de- inking paper sludge on the antagonism between two compost bacteria and Pythium ultimum. FEMS Microbiology Ecology, 52(Suppl 2): 219–227, 2005.
25. BARRIUSO, J, SOLANO, BR, LUCAS, JA, LOBO, AP, VILLARACO, AG, MAÑERO, FJG, Ecology, Genetic Diversity and Screening Strategies of Plant Growth Promoting Rhizobacteria (PGPR). WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Edited by Ahmad I, Pichtel J, Hayat S, 1-17, 2008.
26. ORHAN, E, ESITKEN, A, ERCISLI, S, TURAN, M,
SAHIN,F, Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Scientia Horticulturae, 111 (suppl 1): 38-43, 2006.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1411
ISSN 2229-5518
27. GARCÍA, JAL, PROBANZA, A, RAMOS, B, PALOMINO, MR, MAÑERO, FJG, Effect of inoculation of Bacillus licheniformis on tomato and pepper. Agronomie for Sustainable Development, 24 (Suppl 4):
169-176, 2004.
28. KAYMAK, HC, YARALI,F, GUVENC, I, DONMEZ,
MF, The effect of inoculation with plant growth
Rhizobacteria (PGPR) on root formation of mint (Mentha piperita L) Cuttings, African Journal of Biotechnology, 7 (Suppl 24): 4479-4483, 2008.
29. HAN, HS, SUPANJANI, LEE,KD, Effect of co-
inoculation with phosphate and potassium solubilizing
bacteria on mineral uptake and growth of pepper and cucumber. Plant soil and Environment, 52 (Suppl 3):
130–136, 2006.
30. SUPANJANI, HAN, HS, JUNG, JS, LEE, K. D, Rock
phosphate-potassium and rock-solubilising bacteria as
alternative, sustainable fertilisers. Agronomy for
Sustainable Development, 26 (Suppl 4): 233-240, 2008.
31. HAFEEZ, FY, YASMIN,S, ARIANI,D, MEHBOOB-UR- RAHMAN ZAFAR,Y, MALIK,KA, Plant growth- promoting bacteria as biofertilizer. Agronomy for Sustainable Development, 26:143-150, 2006.
32. CAPPUCCINO,J.C, SHERMAN, N, In Microbiology: A
Laboratory Manual third ed. Benjamin/Cummings
Pub.Co.,New York,pp:125-179, 1992.
33. NAUTIYAL, C.S, MEHTA, S, An Efficient Method for
Qualitative Screening of Phosphate-Solubilizing
Bacteria. Microbiol, 43:51-56, 2001.
34. JACKSON, M.L, Estimation of phosphorus content.
Soil chemical analysis, Printer Hall, New Delhi (India),
1973.
35. BRICK,J.M,BOSTOCK,R.M,SILVERSTONE,S.E, Rapid in situ assay for Indole acetic acid production by bacteria immobilized on nitrocellulose membrane.Appl.Environ.Microbiol,57,535-538, 1991.
36. LOPER, J.E, SCROTH,M.N, Influence of bacterial
sources on indole-3 acetic acid on root elongation of sugar beet. Phytopathology 76,386-389, 1986.
37. DEMUTSKAYA,LN,KALINICHENKO IE,Photometric determination of ammonium nitrogen with the nessler reagent in drinking water after its chlorination, J Water Chem Tech.32(2):90-94, 2010
38. GUGI, B, ORANGE, N, HELLIO, F, BURINI, JF,
GUILLOU, C, LERICHE, F,GUESPIN-MICHAEL ,JF,
Effect of growth temperature on several exported enzymes activities in the psychrotrophic bacterium Pseudomonas fluorescence, J bactriol, 173,3814, 1991.
39. CLARKE H, COWAN ST, Biochemical methods for
bacteriology, J Gen Microbiol 6:187-197, 1952.
40. KANG Y,CHENG J, MEI L,YIN S, Screening and
identification of plant growth promoting
rhizobacteria.Acta microbiologica Sinica,50 (7):853,
2010.
41. BAZZICALUPO, M, FANI, R, Humana Press., Inc., Totowa, N, 112-124, 1995
42. PANDEY, P, KANG, SC, MAHESWARI, DK,Curr Sci,
89(1): 177-180, 1986.
43. ASTCHUL, SF, GISH, W, MILLER, W, MYERS, EV,
LIPMAN, D, J Mol Biol, 215: 403-410, 1990.
44. TAMURA, K, DUDLEY, J, NEI ,M, KUMAR, SM,Mol
Biol Evol, 24, 159601599, 2007.
45. SHARMA, P.D, Fungi and allied organisms, Alpha sciences International Ltd, 2005.
46. GRAHAM PH,VANCE CP, Nitrogen fixation in perspective : An overview of research and extension needs, Field Crops Res, 65:93-106, 1991.
47. RICHARDSON AE, Prosects for using soil microorganisms to improve the acquisitions of phosphorous by plants. Aust J.Plant Physiol, 28:897-
906, 2001.
48. HAAS, D, DEFAGO, G, Biological control of Soil borne
Pathogens by Fluorescent Pseudomonas, Nat Rev
Microbiol,3 (4):307-319, 2005.
49. JAN AT AZAM M,ALI A,HAQ Q, Novel approaches of beneficial Pseudomonas in mitigation of plant disease-an appraisal, J Plant Interact.6(4):195-205, 2011.
50. ANDREWMAGYROSY, Chloroxanthomycin, a fluorescent ,chlorinated ,pentacyclic pyrene from a bacillus sp.Appl, Environ Microbiol,68(8):4095-4101,
2002.
51. GLICK BR, PASTERNAK JJ, Plant growth promoting
bacteria,In: Glick BR, Pasternak JJ , editors, Molecular biotechnology priciples and applications of recombinant DNA. 3rd edn. Washington: ASM Press; p.436-454, 2003.
52. O’SULLIVAN and O’GARA, Traits of
fluorescent Pseudomonas spp. involved in the suppression of plantpathogens Microbiol. Rev,
56 (1992), pp. 662–676,1992.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 12, December-2014 1412
ISSN 2229-5518
IJSER © 2014 http://www.ijser.org