International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 145
ISSN 2229-5518
Fidelity Testing, an Approach to ensure the
Genomic Stability of the Biologically
Hardened Micro Propagated plants
*1Sunitha Panigrahi, 2 Dr.K.Aruna Lakshmi
Abstract— The current work was carried out in continuation to our previous research on hardening of the Micro propagated plants using micro organisms as biological agents. We used single and mixed combinations of microbes and prepared the standard solutions into which the roots of the plantlets were dipped and incubated at the time of Green house stage of the tissue culture. The plants produced in this way gave very good results in terms of their protein content and nutritive value. The question was that weather the plant genetic material was changed due to the use of these microbes. It must be proved that the genome of the plant was conserved and did not get any mutations due to the symbiotic association with the used
micro organisms. The current was focused to prove this aspect using the Fidelity test for the assessment of the quality of the DNA obtained from the progeny. In order to verify the genetic stability the Granine naine variety of banana which was treated with the microorganisms was tested for the fidelity of its genetic material. The work involves the assessment of cloned fidelity of 13 randomly selected hardened plants using 6 ISSR primers.
Index Terms— Hardening, Fidelity Test, Micro propagation, Granine naine, Genetic Material, Conservation.
—————————— ——————————
1 INTRODUCTION
Micro propagation is a reliable technology applied commercially worldwide for large-scale plant multiplication, germplasm conservation, pathogen elimination, genetic manipulations and supply of selected plants [1]. The technology enables the production of large number of plants within a short span of time. The micropropagules thus cultured in-vitro, must then be transferred to the green house or exposed to field conditions [2]. However during this transfer, the mortality rate among the micropropagules is very high as the plants now face various stress conditions such as higher intensity of light, lower humidity levels and soil microorganisms when compared to the sterile in vitro conditions
————————————————
• Sunitha Panigrahi is currently Lecturer in Biotechnology, St.Mary’s college, Hyd,AP, India, E-mail: sritha17@yahoo.com
• Dr.K.Aruna Lakshmi is currently Professor, Dept of
Biotechnology, GITAM University,Vsp,AP, India.
[3].The major obstacle for the success of tissue cultured plants to be used for the production is the loss of these plants due to improper hardening. Thus there were several attempts by the researches to cause the hardening of these plants so as to increase the fertility and productivity. One such method is the use of biological sources like micro organisms in the process of hardening. Several organisms like Piriformospora indica have been used in the hardening of the micro propagated plants like tobacco [4]. The micro-cloned plantlets of Chlorophytum borivilianum registered more than 95% establishment in soil following treatment with various bio-inoculants namely; Glomus aggregatum , Trichoderma harazianum and Piriformospora indica [5] Not only the type of microorganism used but also the concentration of these organisms used also alters the hardening of the plantlets. Sometimes instead of using a single micro organism a combination of micro organisms may have a beneficial effect. It is the end user who
would select the best combination and the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 146
ISSN 2229-5518
concentration of these biological agents in order to obtain maximum yield from the plantlets. Whenever such methods are employed for the development of micro propagated plants there are high chances that the genetic matter of the plantlets may be altered. Soma clonal variations mostly occur as a response to the stress imposed on the plant in culture conditions and are manifested in the form of DNA methylation, chromosome rearrangements, and point mutations. (Phillips et al.1994) [7]. Hence it is an important criterion to check for the Genetic Fidelity to prove that the plants produced are pure lines and are true to type. Molecular techniques are at present powerful and valuable tools used in analysis of genetic fidelity of in vitro propagated plants. Several DNA markers have been successfully employed to assess the genomic stability in regenerated plants including those with no obvious phenotypic alternations [6]. Among the known markers, Inter Simple Sequence Repeat (ISSR) and Random Amplified Polymorphic DNA (RAPD) has been mostly favored. ISSR markers, developed by Zietkiewicz et al. (1994) is based on amplification of a single primer containing a microsatellite “core” sequence anchored at the 3′ or
5′ end by a set of 2–4 purine or pyrimidine residues. This technique is more specific, offers a high degree of reproducibility and a rich level of polymorphism in a relatively simple and low cost procedure, overcoming common criticism against RAPD.
MATERIALS AND METHODS
I. Plant materials and culture condition Rhizome and leaf segments (each approx. 3–5 mm long) of Granine naine variety of banana, procured from the A.G. Biotech Laboratory, (Hyd), India were used as primary explants. Rhizomes were collected from 11-month-old potted green house plants, freed from roots and apical shoot portion and washed thoroughly with tap water to remove soil. Both mature and young leaf segments were used for culture initiation. The young leaves were light green in color, smooth, glabrous. The mature leaves are dark greenish in color, leaf blade 90 cm long and have flat axial surface.
II. Surface Sterilization and Inoculation:
For surface sterilization, explants were treated with
0.1% (w/v) aqueous HgCl2 solution for 3–5 min and rinsed thoroughly in sterile double distilled water and blot dried. The sterilized explants (rhizome and leaf segments) were cut obliquely to remove mature or damaged parts and placed horizontally in MS (Murashige and Skoog, 1962) nutrient agar medium (20 ml/tube and 50 ml/bottle) containing various concentrations and combinations of plant hormones along with other defined and undefined plant growth regulators (PGR) under aseptic condition in a laminar hood. The pH of the medium was adjusted to 5.8 before adding 0.8% agar (Bacteriological grade, Hi-media) and prior to autoclaving at 1.1 kg cm2 pressure (121 °C) for 15 min. All the cultures were incubated under cool white fluorescent light (16h photoperiod, 40 m m−2 s−1, Philips, India) at 25 ±
2.0 °C and 60–70% relative humidity (RH).
III. Callus Induction and plant regeneration Effects of various auxins namely 2, 4-D and α- Napthalene Acetic Acid (NAA), on callus induction were investigated. Callus induction was achieved on MS basal medium supplemented with various concentrations of 2, 4-D (9.05–18.10 μM), NAA (1.34–16.11 μM), either alone or in combinations with IBA (1.12–8.88 μM) and 10% CW. (CaP). The regenerated plantlets were transferred to full, half and one-fourth strength MS medium containing 2.46–9.84 μM IBA with 2% sucrose for rhizogenesis. Each experiment was repeated thrice with 10 replications. The regenerants were initially maintained in half strength liquid MS medium for 12 days under high humidity condition (95% RH). The well rooted shoots were gently removed from the culture vessels, washed under tap water to remove traces of medium, and transferred to plastic pots containing different sterilized potting mixtures, viz. soil : vermiculate: sand (2:1:1); soil : vermiculate : sand (2:2:1); garden soil : organic manure (1:1); sand : soil: compost (2:2:1); and sand
: soil : compost (2:1:1); 200 samplings of Grand
Nain were taken from AG biotech. They were divided equally into four groups. The first 50
plants were taken as the control. To the next 50,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 147
ISSN 2229-5518
consortium was added having a concentration of
2.5%. The consortium consisted of PSB, Acetobacter, Pseudomonas, VAM, Rhizobium and Azospirullum. The next 50 were AG Biotech processes plants and were taken as a control, the last 50 were AG biotech processed containing 2.5% consortium. The plants were then kept in the green house for three months and the root length, shoot length, number of leaves and primodes were measured every week and are recorded. The plants are maintained under green house conditions (27–
30 °C, 85% RH) for 60 days.
IV. DNA extraction and ISSR amplification
The Cetyl trimethyl ammonium bromide (CTAB) method, with certain modifications in the extraction procedure according to Khanuja et al. (1999) was used to extract genomic DNA from leaves and rhizomes of mother plant (used as the source of explants for tissue culture) as well as from randomly selected 10 plants of each was extracted. DNA was isolated at least three times from each plant and the quantity and quality of extracted DNA samples were estimated by comparing band intensities on 0.8% agarose gel. Genomic DNA was then PCR amplified using ISSR primers (3′ anchored). The annealing temperature was found to vary according to the base composition of the primers. Total 17 ISSR primers were screened initially out of which 6 ISSR markers were found to generate reproducible, unambiguous amplification profiles. Amplification reaction volumes were 25 μl, each containing 25 ng template genomic DNA, 1× PCR buffer, 130 μM dNTPs, 50 mM MgCl2, 0.3 μl (3 U/μl) Taq DNA polymerase, 0.5 μl (50 μM) primer. Amplifications were performed in a thermocycler (Perkin elmer
2400 gene Amp PCR system) programmed for an initial denaturation at 94 °C for 4 min followed by
40 cycles of 1 min denaturation at 94 °C, 1 min annealing at a temperature 2 °C lower than melting point for each primer and 2 min extension at 72 °C with a final extension of 72 °C for 10 min (Wolfe et al., 1998). Control reactions containing water in place of genomic DNA were also performed along side in order to verify absence of contamination. The PCR products obtained were separated on 2%
agarose gel , stained with ethidium bromide (0.5
μg/ml) and documented in a gel documentation system (Gel Doc1000 Biorad). A 1200 bp DNA ladder was included in the gels as a size reference. Only those bands that showed consistent unambiguous amplification were considered. All the reagents for PCR analysis were procured from University of Agricultural Sciences, GKVK, Bangalore, India.
V. Data analysis
The data from similarity indices were used to obtain distance matrix depicting the genetic relatedness of each randomly selected embryo derived clones with the mother plant.
RESULTS AND DISCUSSION
Initial induction of callus was noted after 15–18 days of inoculation on MS medium containing various concentrations of auxins. Mature leaf segments failed to survive. Young leaf explants and rhizomatous segments cultured on MS medium supplemented with 13.57 μM 2,4-D, 8.88 μM BA and 10% CW showed significantly (p <
0.05) higher induction of callus. Within 6 weeks of culture, callus formation was recorded from both explant types.
Data recorded after 6 weeks culture. CIM: callus induction medium. Each treatment was repeated three times. Basal medium: MS + 3% sucrose +
0.8% agar.
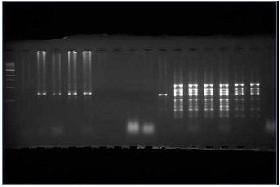
Eight randomly selected well-developed healthy tissue cultured plants with all the four combinations produced clear, reproducible amplification profiles when screened using 6 ISSR primers (of 13 primers tested) (Fig. 2). A total 32 unambiguous bands in the size range 200–1000 bp were scored from 6 primers out of which 29 were present in donor plant as well as in all the regenerants and rest 3 bands were polymorphic . The number of scorable bands varied from 4 (BG04) to 7 (BG-01) with an average of 5.33 bands/primer.
ISSR profile using primer BG-01 of hardened tissue cultured plant of banana resolved on 2.0% agarose gel.
There as genetic variations seen in some micropropagated plants as DNA methylation polymorphism was evaluated in micropropagated banana (Musa AAA cv. ‘Grand naine’) [8]. There were minor morphological variations recorded in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 148
ISSN 2229-5518
the micropropagated plants, the developed ISSR profiles were typical to that of mother plant of Ochreinauclea missionis plantlets [9]. Differential expression of micro RNA’s in conventional and micropropagated strawberry plants stated that there were less genetic variation seen in micropropagated plants than in conventional method [10].
CONCLUSION
In our study the biohardened micropropagated plants showed varied increase in the protein, nutrient, carbohydrate and phenol contents which had raised the thought of mutations to be occurred in the plant genome, hence, fedility test was conducted and proved that the genetic material was same as mother plant and it was true type.
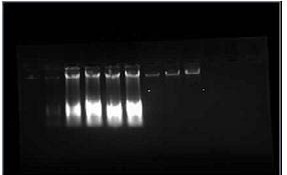
Figure 1: The bands of the DNA extracted from 4 plants (Banana control, Banana consortium, Banana AG control, Banana AG consortium) comparing the band intensities on
0.8% agarose gel.
Figure 2: ISSR profile using primer BG-01 of hardened plants and parent plant of G.naine variety of banana resolved on 2.0% agarose gel.
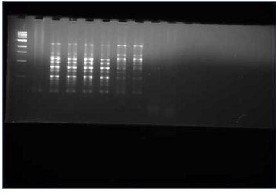
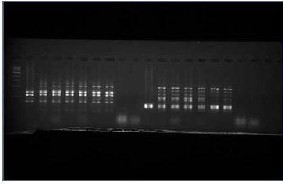
Fig-3 the bands obtained shows the true type of genetic material present
Fig-4: the bands obtained shows the true type of genetic material present
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 149

ISSN 2229-5518
. Fig-5: The report issued by AG Biotech that shows that the plants hardened after the use of Microbes were still True to Type of the parent plant that proved the Fidelity of the plants
REFERENCES
[1] http://www.springer.com/life+sciences/plant+sciences/boo k/978-1-62703-073-1
[2] Helix May_June 2013 volume 3,
[3] Preece JE, Sutter EG, Micropropagation, 1990, Acclimatization of micropropagated plants to the greenhouse and field, pg. 71-
93.
[4] Piriformospora indica: a new biological hardening tool for micropropagated plants http://www.ncbi.nlm.nih.gov/pubmed/10585552
[5] Biological hardening and genetic fidelity testing of micro- cloned progeny of Chlorophytum borivilianum Sant. et Fernand. African Journal of Biotechnology (ISSN: 1684-5315) Vol 7 Num 8 DOI:jb08176 http://www.researchgate.net/publication/27798241_Biologic al_hardening_and_genetic_fidelity_testing_of_micro- cloned_progeny_of_Chlorophytum_borivilianum_Sant._et_Fe rnand
[6] Genetic fidelity in micropropagated plantlets of Ochreinauclea missionis an endemic, threatened and medicinal tree using
ISSR markers. M. Chandrika and V. Ravishankar Rai*
African Journal of Biotechnology Vol. 8 (13), pp. 2933-2938, 6
July, 2009
[7] Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. P.Joshi and V.Dhawan*
Biologia PlantarumVol51(1),pg no:22-26,March 2007
[8] Detection of DNA methylation changes in micropropagated banana plants using methylation-sensitive amplification polymorphism (MSAP), Plant Science, Volume 161, Issue 2, July 2001, Pages 359–367
[9] Genetic fidelity in micropropagated plantlets of Ochreinauclea
missionis an endemic, threatened and medicinal tree using ISSR markers M Chandrika, VR Rai*, African Journal of Biotechnology. ISSN: 1684-5315
[10] MicroRNA expression profiles in conventional and micropropagated strawberry (Fragaria × ananassa Duch.) plants, He Li, Zhihong Zhang, Feifei Huang, Linlin Chang, Yue Ma*, Plant Science Reports, vol 28, Issue 6, June 2009, Pg no: 891-902
IJSER © 2013 http://www.ijser.org




