International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1602
ISSN 2229-5518
1 Chemistry Department, Faculty of Science, Port Said University, Port Said, Egypt.
2 Chemistry Department, Faculty of Science, Suez University, Suez, Egypt.
3 Chemistry Department, Faculty of Petroleum and Minning Engineering, Suez University, Suez, Egypt. Email:
asmaa_semida88@yahoo.com
In this work novel organic based compounds, nitrogen heterocycles were synthesized and their antimicrobial and anticancer activities. Anew series of nitrogen heterocycles (3 and 7) containing ferrocene ring were prepared via the reaction of 1-acetyl ferrocene (1) with thiosemicarbazide to give N-substituted thiourea (2), followed by cyclization with ethyl chloroacetate and phenacyl bromide. Condensation of 3 with aromatic aldehyde, yielded arylidene derivatives(5). Acetyation of compounds 3, 5 and 7 with acetic anhydride gave the corresponding N-acetyl derivatives (4, 6 and 8). The structures of the synthesized compounds were confirmed by IR, 1H-NMR, 13C-NMR, MS and elemental analysis. Antimicrobial and antitumor activities of some synthesized compounds have been investigated. The two compounds, selected as potential agents hepatocellular carcinoma (HCC) were then evaluated in vitro for their biological activity on HCC- derived cell lines(The compounds show a promising inhibitory growth efficacy (IC50 = 2.61and 2-
69µg) with compared standard antitumor drug (IC50 = 4.60 µg).
The hydantoin, imidazolidene-2, 4-dione, scaffold is an important structural component that is present in a number of natural products [1,2] and chemotherapeutic important compounds [3-5]. Hydantoins substituted at the C-5 position exhibit a wide range of pharmacological activities including antiarrhythmic and antihypertensive [6], antiviral [7], antineoplastic [8], antitumoral [9], anticonvulsant agents [10], antidepressant [11] and platelet aggregation inhibitory activities [12]. Phenylhydantoins have attracted great interest because of their beneficial biological properties such as:
antiepileptic [13], herbicides [14] and fungicides [15].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1603
ISSN 2229-5518
To the best of our knowledge, incorporation of ferrocene fragment into an organic compound
often led to unexpected biological activity, which is due to their different membrane permeation properties and anomalous metabolism. Many ferrocenyl-based compounds display interesting cytotoxic, antitumor, antimalarial, antifungal and DNA-cleaving activity [16]. Recently, some new ferrocenyl-substituted heterocyclic compounds have been reported as potential pharmaceuticals [17,
18]. Moreover, the stability and non-toxicity of the ferrocenyl moiety is of particular interest
rendering such drugs compatible with other treatment [19].
In view of these observations and as a part of our ongoing program devoted to the synthesis of diverse heterocycles as antimicrobial/ anticancer agents [20, 21], in this study, we report herein synthesis and preliminarily in vitro antimicrobial activity and cytotoxicity of thiohyantoin derivatives substituted with phenyl or arylidene and ferrocene moieties.
2.1 Chemistry
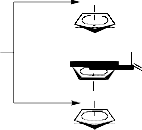
The synthesis of thiohyantoin-scaffold bearing phenyl or arylidene and ferrocenyl terminals (5-
8) is depicted in Schemes 1, 2. The key starting materials 1-acetylferrocene thiosemicarbazone (2) was synthesized starting from 1-acetylferrocene (1) following modified literature procedure [3], via refluxing of an ethanolic solution of 1-acetylferrocene and thiosemicarbazide. Treatment of the thiourea derivative (2) with ethyl chloroacetate in the presence catalytic amount of fused sodium acetate in methanol afforded the corresponding ferrocenyl-thiohyantoin, 3-[(ferrocene-1- ylethylidene)amino]-2-thioxo-imidazolidine-4-one (3) (Schemes 1). Furthermore, acetylferrocene
thiosemicarbazone (2) undergoes coupling reaction with phenacyl bromide in the presence of fused sodium acetate in acetic acid under reflux to give the corresponding 4-phenyl-3-[(ferrocene-1- ylethylidene)amino]-imidazolidine-2-thione (7) which was converted to 1-acetyl-3-[(ferrocene-1- ylethlidene)amino]-4-phenyl-imidazolidine-2-thione (8) via the acylation with acetic anhydride under reflux conditions (cf. Schemes 1).
Ferrocenyl-thiohyantoin (3) was either converted into the 1-acetyl-3-[(ferrocene-1-ylethylidene) amino]-4-hydroxy-imidazolidine-2-thione (4) through boiling in acetic anhydride or reacted with two different aromatic aldehydes (a, b) in piperidine under fusion to yield the corresponding ferrocenyl-thiohyantoin arylidenes, 3-[(ferrocene-1-ylethylidene) amino]-2-thioxo-5-arylidene- imidazolidine-4-ones (5a, b) (see Scheme2). The ferrocenyl-thiohyantoin arylidenes (5a, b) were further acylated with acetic anhydride to afford the corresponding 1-acetyl-2-thioxo-3-[(ferrocene-
1-ylethylidene)amino]-5-arylidene-imidazolidine-4-ones (6a, b).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1604
ISSN 2229-5518
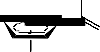
CH3
![]()
![]()
CH3 S
![]()
![]()
CH3 S
O
![]()
![]()
Fe TSC Fe
MeOH/reflux
NNH-C-NH2
![]()
ClCH2COOEt
MeOH/AcONa
![]()
![]()
![]()
N N N-H Fe
O
![]()
PhCOCH2Br
AcOH/AcONa
![]()
![]()
CH3 S
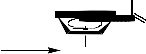
![]()
CH3 S
![]()
Fe
![]()
![]()
N N N-H C6H5
Ac2O
reflux
![]()
Fe
![]()
![]()
![]()
N N N
O C6H5
CH3
Schemes 1
![]()
![]()
CH3 S
![]()
![]()
CH3 S
Ac2O
![]()
![]()
![]()
N N N
Fe O
CH3
![]()
![]()
N N N-H
reflux HO
![]()
![]()
Fe (4) CH3 S
![]()
![]()
![]()
CH3 S O
O
ArCHO
Piperidine reflux
Fe
![]()
![]()
N N N-H O
Ar Ac2O
reflux
![]()
![]()
![]()
N N N Fe
O
CH3
Ar
(a, Ar = C6H5; b, Ar = 2-OH-C6H4)
Schemes 2
2.2 Characterization of the Ferrocenyl-thiohyantoins
The ferrocenyl-thiohyantoins (3-8) were prepared in high yields, gave satisfactory C, H, N and S elemental analyses, which are consistent with the proposed formula. The IR spectra of compounds (3-5) exhibited characteristic peaks at 3210-3227, 1710-1728 and 1427-1462 cm-1 due to thiohyantoin NH, carbonyl and thiocarbonyl moieties, respectively. Interestingly, the vibration at the region 1632±5 cm−1 may be assigned to the imine (C=N) stretch. The NH stretches in the FTIR spectra of the - were missing from the spectra of compounds (6, 8) indicating acylation of the thiohyantoin nitrogen and replacement of H by the acetyl group. This was further supported by the appearance of new strong peak at ca. 1712±4 cm−1 in the spectra of (6, 8), ascribed to acetyl C=O
group.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1605
ISSN 2229-5518
2.3 Biological assessment
Antibacterial activities were investigated using agar well diffusion method. The activity of tested samples were studied against the bacillus subtilis (RCMB 000 107) and staphylococcus aureus (RCMB 000 106) as gram positive bacteria, while Escherichia coli (RCMB 000 103) and pseudomonas aeruginosa (RCMB 000 102) as gram negative bacteria [22, 23].The zone of inhibition was measured in mm. and was compared with standard drug. DMSO was used as a blank and ciprofloxacin was used as antibacterial standard. All the compounds were tested at 10 and 50 mg
concentrations. The date is summarized in table 1, and show that all compounds display certain antibacterial activity.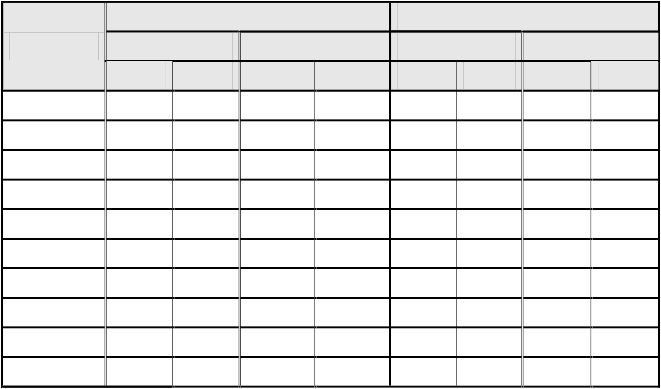
Gram positive bacteria Gram negative bacteria
Compd. No.
Bacillus Sub. Staphylococcus E-Coli Pseudomonas Sp.
10 mg | 50 mg | 10 mg | 50 mg | 10 mg | 50 mg | 10 mg | 50 mg | |
2 | - | 4 | 6 | 10 | - | 7 | 2 | 9 |
3 | 2 | 7 | 3 | 8 | - | 9 | 5 | 9 |
4 | - | 1 | - | 2 | - | 4 | - | 1 |
5a | 9 | 13 | 8 | 14 | 8 | 14 | 9 | 15 |
5b | 5 | 12 | 6 | 11 | 7 | 10 | 4 | 11 |
6a | 6 | 10 | 7 | 13 | 7 | 13 | 5 | 12 |
6b | 5 | 11 | 5 | 10 | 4 | 10 | 6 | 11 |
7 | 8 | 11 | 6 | 10 | 3 | 7 | 8 | 11 |
8 | 7 | 9 | 3 | 8 | 5 | 9 | 7 | 10 |
Ciprofloxacin | 11 | 15 | 12 | 16 | 10 | 16 | 13 | 18 |
Tested samples were screened separately using agar well diffusion method [22, 23] against various fungi (Aspergillus Nigaer and Penicillium Sp.). The inhibition zone was measured in mm. and was compared with fluconazole as standard drug, and using DMSO as a blank as antifungal activity. All
the compounds were tested at 10 and 50 mg concentrations. The data are listed in table 2.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1606
ISSN 2229-5518
![]()
Compd. No. | Aspeargillus Nigaer | Penicllium Sp. | ||
Compd. No. | 10 mg | 50 mg | 10 mg | 50 mg |
2 | - | 5 | - | 7 |
3 | 2 | 8 | - | 9 |
4 | 1 | 7 | - | 3 |
5a | 9 | 14 | 8 | 16 |
5b | 5 | 11 | 9 | 14 |
6a | 6 | 13 | 7 | 13 |
6b | 4 | 9 | 6 | 10 |
7 | 5 | 10 | 6 | 11 |
8 | 4 | 8 | 5 | 9 |
Fluconazole | 11 | 18 | 13 | 19 |
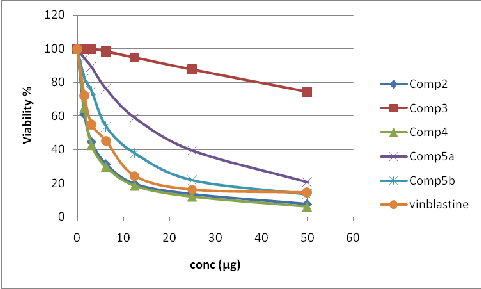
In this study, the anticancer activity of the 9 synthesized compounds bearing ferrocene moiety has been evaluated on human cancer cell lines, representing liver cancer. The cytotoxic activities of prepared compounds were tested against HepG-2 cell line according to method of Masmann and Vijayen et al [24, 25].The inhibitory activity against liver carcinoma cells (HepG-2) was detected by using different concentrations of tested samples (50, 25, 12.5, 6.25, 3.125, and 1.65
µg) and the viability cells (%) were determined by colorimetric method. The IC50 was calculated
from table 3 and figures 1 and 2.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1607
ISSN 2229-5518
![]()
Sample Conc. (µg) | Viability (%) | |||||||||
Sample Conc. (µg) | 2 | 3 | 4 | 5a | 5b | 6a | 6b | 7 | 8 | Vinblastine Standard |
50 | 7.64 | 74.32 | 5.97 | 20.58 | 13.39 | 70.86 | 26.92 | 32.56 | 12.71 | 14.38 |
25 | 13.55 | 87.63 | 11.94 | 39.47 | 21.83 | 87.42 | 42.13 | 68.47 | 29.46 | 16.13 |
2.5 | 19.62 | 94.78 | 18.38 | 58.94 | 37.82 | 95.08 | 79.48 | 83.19 | 72.65 | 24.25 |
6.25 | 31.43 | 98.39 | 29.72 | 76.22 | 53.87 | 99.12 | 88.17 | 92.51 | 84.16 | 45.13 |
3.125 | 44.61 | 100.0 | 42.53 | 89.16 | 74.91 | 100.0 | 95.08 | 47.89 | 93.58 | 55.00 |
1.65 | 60.98 | 100.0 | 64.78 | 94.38 | 82.54 | 100.0 | 98.72 | 99.13 | 97.84 | 72.13 |
0.00 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.00 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1608
ISSN 2229-5518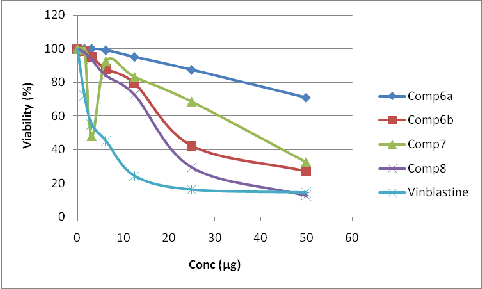
The results of 50% inhibitory concentration (IC50 ) date are summarized in table 4.
Table 4: IC50 (µg) values of nitrogen heterocycles after 24 h continuous exposure of tumor cell line
Compound No. | 2 | 3 | 4 | 5a | 5b | 6a | 6b | 7 | 8 | Vinblastine Standard |
Tumor type/Cell line HepG-2 | 2.61 | >50 | 2.69 | 18.20 | 7.76 | >50 | 22.40 | 37.9 | 19.1 | 4.60 |
The IC50 value is the concentration that induces 50% growth inhibition compared with untreated control cells.
According to the results of cell culture studies, the compounds 2 and 4 showed antitumor activity than the standard antitumor drug against HepG-2 cell line.
In comparison with standard antitumor drug vinblastine, all another tested compounds have cytotoxic and antitumor activity less than standard antitumor drug against hepatocellular carcinoma
cell line (HepG-2).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1609
ISSN 2229-5518
A new series of nitrogen heterocycles containing ferrocene ring were prepared. The structures of these compounds were confirmed by IR, 1H-NMR, 13C-NMR, MS and elemental analysis. Anticancer activities of synthesized compounds were evaluated on liver cancer cell lines. As a result of the cell culture studies, all of the compounds have shown anticancer activity for liver cancer cells. In conclusion, some nitrogen heterocycles compounds might be potentially useful in the field of cancer treatment, finally compounds 2, 4 and 5b can be suggested as potent candidates
for liver cancer drug.
All starting materials, reagents, and solvents were purchased from commercial suppliers. Melting points were measured in open capillary tubes on a MEL-TEMP II melting points apparatus and uncorrected. The NMR spectra were recorded on a BRUKER 300 BB instrument at room temperature, operating at 300 MHz for 1H and 75.075 MHz for 13C. The chemical shifts were recorded as δ values in ppm units downfield of tetramethylsilane used as internal standard. The IR spectra were recorded on a FT/IR – 4200 spectrometer in KBr pellets. Mass spectra were obtained
on a Joel JMS D-300 spectrometer operating at 70 eV. The elemental analyses were performed on a
Perkin Elmer 2400 series II CHNS/O elemental analyzer.
1-[( Ferrocene-1-ylethylidene) amino]-thiourea (2)
A mixture of 2-acetylferrocine (1, 0.01mole) and thiosemicarbazide (0.01mole) in methanol (50 mL) was heated under reflux for 4 h, and then cooled. The solid formed was filtered off, dried and purified by recrystalization from methanol to give 2 as pale red crystals, yield 78%, m. p.
175oC. IR (KBr): 3410, 3128 (NH2 ), 3218 (NH), 1635 (C=N), 1431 (C=S) cm-1. 1H-NMR (DMSO
– d6 ): δ 2.19 (s, 3H, CH3 ), 3.32 (s, 2H, NH2 ), 4.17 – 4.80 (m, 9H, H–ferrocene ring), 9.95 (s, 1H, NH) ppm. 13C-NMR (DMSO -d6 ): δ 178.42 (C = S), 151.03 (C = N), 83.55, 72.59, 71.16, 70.21,
70.10, 70.00, 69.83, 69.49, 68.19, 67.73 (C–ferrocine ring), 15.52 (CH3 ) ppm. MS: m/z (%) = 303 (M+ + 2, 5.60), 302 (M+ + 1, 18.30), 301 (M+, 79.00), 300 (M+- 1, 10.00), 285 (10.40), 284 (49.70),
283 (24.50), 269 (2.50), 268 (1.80), 267 (3.8), 266 (2.10), 259 (1.60), 258 (1.00), 249 (2.60), 248 (8.40), 247 (6.60), 237 (1.70), 236 (4.80), 235 (4.40), 234 (2.00), 228 (10.80), 227 (11.90), 226 (17.10), 219 (4.80), 218 (16.40), 217 (3.60), 212 (8.40), 211 (11.30), 202 (5.30), 201 (2.60), 197 (5.00), 196 (35.20), 195 (17.50), 194 (22.40), 193 (15.30), 186 (14.30), 185 (56.20), 184 (27.0), 178 (15.60), 177 (17.30), 176 (6.10), 161 (5.80), 160 (14.00), 159 (7.80), 154 (9.80), 153 (25.70), 152 (16.90), 151 (7.90), 148 (5.50), 147 (5.10), 146 (7.10), 138 (3.20), 137 (32.80), 136 (21.70), 135
(8.50), 129 (28.10), 128 (15.00), 127 (6.00), 122 (11.20), 121 (77.30), 120 (20.60), 119 (9.40), 107
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1610
ISSN 2229-5518
(8.30), 106 (6.30), 105 (10.50), 104 (7.20), 97 (26.60), 96 (8.00), 95 (14.00), 94 (17.30), 89 (15.40),
88 (9.10), 81 (16.90), 80 (16.40), 74 (7.80), 77 (8.50), 66 (5.50), 65 (18.10), 64 (13.70), 63 (14.30),
57 (10.20), 56 (100), 55 (21.60), 54 (9.70), 51 (7.30). Anal. Found: C, 51.73; H, 4.89; N, 13.83; S,
10.48. C13 H15 N3 FeS requires: C, 51.83; H, 4.98; N, 13.95; S, 10.63.
3-[(Ferrocene-1-ylethylidene) amino]-2-thioxo-imidazolidine-4-one (3)
A mixture of (2, 0.01mole), ethyl chloroacetate (0.01mole) and fused sodium acetate (0.03mole) in methanol (30 mL) was heated under reflux for 3-4 h. The solid formed after cooling was filtered off, washed with hot water, dried and purified by recrystallization from methanol to give 3 as pale red crystals, yield 73%, m. p. 240oC. IR (KBr): 3210 (NH), 1728 (C = O), 1627 (C = N), 1427 (C = S) cm-1. 1H - NMR (DMSO – d6 ): δ 2.23 (s, 3H, CH3 ), 3.81 (s, 2H, NCH2 CO),
4.17 – 4.68 (m, 9H, H-ferrocene ring), 10.30 (S, 1H, NH) ppm. 13C–NMR (DMSO – d6 ): δ 178.11
(C = S), 169.31 (C = O), 151.31 (C = N), 83.24, 72.58, 70.41, 70.10, 69.78, 69.64, 67.70 (C –
ferrocene ring), 33.13(NCH2 CO), 16.21(CH3 ) ppm. MS: m/z(%)= 343 (M++ 2 , 8.70), 342 (M+ + 1,
23.10), 341 (M+, 100), 340 (M+- 1, 22.40), 277 (4.50), 276 (16.40), 275 (8.70), 268 (3.10), 267 (5.20), 249 (5.90), 248 (11.50), 247 (10.80), 237 (5.90), 236 (15.00), 235 (5.60), 234 (15.00), 232 (5.90), 227 (7.30), 226 (8.70), 225 (4.50), 212 (7.00), 211 (8.70), 210 (5.60), 203 (7.70), 202 (34.30), 201 (18.20), 199 (7.30), 198 (3.80), 187 (5.90), 186 (10.10), 185 (23.10), 184 (11.50), 179 (5.2), 178 (5.60), 168 (5.60), 167 (3.50), 166 (11.50), 162 (22.40), 161 (14.00), 160 (9.80), 147 (8.40), 146 (9.40), 145 (6.60), 135 (5.90), 134 (11.50), 132 (7.70), 122 (4.50), 121 (53.50), 120 (9.80), 119 (8.40), 113 (11.90), 112 (4.90), 107 (5.90), 106 (5.90), 105 (7.70), 98 (2.80), 97 (11.20),
96 (6.30), 92 (5.60), 91 (14.00), 89 (5.20), 79 (3.50), 78 (8.00), 77 (8.70), 65 (13.90), 64 (12.90), 63 (8.70), 57 (2.80), 56 (56.60), 55 (9.10), 51 (5.60). Anal. Found: C, 52.67; H, 4.38; N, 12.28; S,
9.29.C15 H15 N3 Fe OS requires: C, 52.78; H, 4.40; N, 12.32; S, 9.38.
5-Arylidene-3-[(ferrocene-1-ylethylidene)amino]-2-thioxo-imidazolidine-4-one (5a, b)
A mixture of (3, 0.01mole), aromatic aldehydes (such as benzaldehyde and 4- methoxybenzaldehyde) (0.01mole) and piperidine (1mL) was fused on a hot-plate at 120-130oC for
1h. The reaction mixture was cooled and acidified with dilute hydrochloric acid (1N). The crude product was filtered off, washed with water, dried and purified by recrystallization from ethanol to give 5.
5-Benzylidene-3[(ferrocene-1-ylethylidene)amino]-2-thioxo-imidazolidine-4-one (5a) as brown crystals, yield 63%, m. p. 160oC. IR (KBr): 3221 (NH), 1710 (C = O), 1637 (C = N), 1605, 1589 (C
= C), 1446 (C = S) cm-1. 1H–NMR (DMSO– d6 ): δ 2.31 (s, 3H, CH3 ), 4.21 – 4.81 (m, 9H, H –
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1611
ISSN 2229-5518
ferrocene ring), 7.44 – 7.88 (m, 6H, Ar- H and H – olefinic), 10.61 (s, 1H, NH) ppm.13C–NMR
(DMSO – d6 ): δ 167.77 (C = S), 164.77 (C = O), 152.31 (C = N), 138.26, 136.64, 134.26, 130.29,
129.71, 128.86, 127.79, 124.06, 120.71 (C – aromatic and C – olefinic), 68.01, 69.75, 69.74, 69.89,
70.17, 70.26, 70.51, 70.75, 72.21, 82.82 (C – ferrocene ring), 16.56 (CH3 ) ppm. MS: m/z (%) =
430 (M+ + 2, 11.30 ), 429(M+ + 1, 9.30 ) , 428 (M+, 17.35 ), 427(M+-1, 7.80), 404(6.30), 403(9.20),
227(8.30), 226(10.70), 225(3.50), 212(6.00), 211(7.80), 210(4.60), 203(8.70), 202(32.300,
201(17.20), 199(6.30), 198(4.80), 187(5.60), 186(9.10), 185(22.10), 184(10.50), 179(4.8),
178(6.60), 168(5.50), 167(3.30), 166(10.50), 162(20.40), 161(14.00), 160(7.80), 147(7.20),
146(8.30), 145(5.60), 135(17.20), 134(100), 132(6.80), 122(3.50), 121(50.60), 120(8.90),
119(8.30), 113(12.80), 112(3.80), 107(6.50), 106(4.90), 105(6.70), 98(2.30), 97(10.30),
96(5.30),92(5.60), 91(16.30), 90(34.20), 89(6.30), 79(3.30), 78(10.20), 77(42.50), 65(13.20),
64(17.10), 63(18.50), 57(3.60), 56(57.20), 55(8.10), 51(15.30). Anal. Found: C, 61.58; H, 4.01; N,
9.71; S, 7.37. C22 H18 N3 FeOS requires: C, 61.68; H, 4.21; N, 9.81; S, 7.47.
5-(2-Hydroxy)benzylidene-3[(ferrocene-1-ylethylidene)amino]-2-thioxo-imidazolidine-4-one (5b) as brown crystals, yield 61%, m. p. 165oC. IR (KBr): 3227 (NH), 1713 (C = O), 1632 (C =N),
1608, 1593 (C = C), 1445 (C = S), 1095, 1052 (C = O) Cm-1. 1H–NMR (DMSO – d6 ): δ 2.32 (s, 3H, CH3 ), 4.23 – 4.80 (m, 9H, H - ferrocene ring), 6.85 - 7.87 (m, 5H, Ar - H and H– olefinic), 10.71 (s, 1H, NH) and 11.35(s, 1H, OH) ppm. 13C-NMR (DMSO – d6 ): δ 168.30 (C
=S), 165.33 (C = O), 153.20 (C – O), 152.30 (N – C = N), 138.24, 136.63, 134.25, 130.28,
129.70, 128.83, 127.78, 120.73 (C – aromatic and C – olefinic), 82.80, 72.22, 70.52, 70.25,
70.16, 69.86, 69.73, 69.34, 68.02 (C – ferrocene ring), 16.53 (CH3 ) ppm. Anal. Found: C,
59.37; H, 3.95; N, 9.22; S, 7.03. C22 H18 N3 FeO2 S requires: C, 59.46; H, 4.05; N, 9.46; S, 7.21.
4-Phenyl-3[(ferrocene-1-ylethylidene)amino]-imidazolidine-2-thione (7)
A mixture of (2 ,0.01mole), phenacyl bromide(0.01mole) and fused sodium acetate (0.03mole ) in glacial acetic acid and (30 mL) was heated under reflux for 2-3 h, then cooled and poured into water. The resulting solid was filtered off, washed with water, dried and purified by recrystallization from ethanol to give 7 as red crystals, yield 64%, m. p. 220oC – IR (KBr): 3309 (NH), 1631 (C = N), 1602, 1585 (C = C), 1442 (C = S) cm-1, 1H-NMR (DMSO – d6 ): δ 2.23 (s,
3H, CH3 ), 4.18 – 4.63 (m, 9H, H – ferrocene ring), 7.26 –7.88 (m, 6H, Ar – H and H –
imidazolidine ring), 10.91 (s, 1H, NH) ppm.13C – NMR (DMSO - d6 ): δ 170.35 (C = S), 151.10 (N
= C – N), 149.01 (=C– N), 135.44, 129.04, 127.84, 125.96, 103.98 (C–aramatic and C–
imidazolidine), 84.43, 69.90, 69.45, 66.96 (C – ferrocene ring), 15.79 (CH3 ) ppm. MS: m/z (%)
=403 (M+ + 2, 7.50), 402 (M+ + 1, 6.50), 401 (M+, 16.30 ), 387(6.20), 386(7.20), 358(6.10),
357(7.70), 228(11.80), 227(13.80), 226(19.300, 219(5.30), 218(17.30), 217(4.50), 212(6.40),
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1612
ISSN 2229-5518
211(13.20), 202(5.10), 201(3.60), 197(5.30), 196(34.20), 195(18.20), 194(21.30), 193(16.20),
186(3.20), 185(56.20), 184(26.30), 178(15.60), 177(16.20), 175(7.20), 161(5.80), 160(13.00),
159(6.80), 154(6.80), 153(23.20), 152(16.80), 151(6.90), 148(4.90), 147(5.20), 146(8.20),
138(2.20), 137(31.20), 136(20.80), 132(10.10), 131(18.50), 128(13.10), 127(5.50), 122(10.50),
121(76.20), 120(14.60), 119(8.30), 107(6.30), 106(5.80), 105(11.30), 104(7.30), 103(23.20),
102(11.70), 92(11.30), 91(17.20), 89(61.30), 88(6.10), 78(7.80), 77(18.50), 76(11.20),
66(4.50),65(11.30), 64(11.20), 63(22.20), 57(11.30), 56(100), 55(23.60), 54(8,70), 51(7.20). Anal. Found: C, 62.73; H, 4.64; N, 10.33; S, 7.83. C21 H19 N3 FeS requires: C, 62.84; H, 4.74; N, 10.47; S,
7.98.
1-Acetyl-3-[(ferrocene-1-ylethylidene) amino]-4-hydroxy imidazolidine-2-thione (4)
1-Acetyl-2-thioxo-3-[(ferrocene-1-ylethylidene) amino]-5-arylidine- imidazolidine-4-ones (6a, b)
1-Acetyl-3-[(ferrocene-1-ylethylidene) amino]-4-phenyl-imidazolidine-2-thione (8)
A solution of 3, 5 and 7 (0.01mole) in acetic anhydride (20 ml) was heated under reflux for
2 hr, then cooled and poured into ice-water. The resulting solid was filtered off, washed with water, dried and purified by recrystallization from benzene to give 4, 6 and 8.
Compound 4 as pale red crystals, yield 58%, m. p. 150oC. IR (KBr): 3460 - 2980 (br. OH),
1728 (C = O), 1635 (C = N), 1462 (C = S), 1221, 1095 (C = O) cm-1. 1H-NMR (DMSO - d6 ): δ 2.13 (s, 3H, COCH3 ), 2.24 (s, 3H, N = C - CH3 ), 3.32 (br. s, OH), 4.14 – 4.64 (m, 10H, H – ferrocene ring and H – imidazolidine) ppm.
13C–NMR (DMSO – d6 ): δ 165.85 (C = S), 164.24 (C = O), 151.30 (-C = N), 150.23 (C – OH),
91.45 (CH of imidazolidine), 63.96, 68.07, 67.92, 67.27, 67.26 (C – ferrocene ring), 23.40, 23.16 (2
× CH3 ) ppm. MS:m/z (%) = 385 (M+ + 2, 7.80), 384 (M+ + 1, 21.30), 383 (M+, 32.30), 343 (6.80),
342 (36.20), 341 (100), 340 (21.40), 277 (4.30), 276 (16.50), 257 (8.30), 268 (3.11), 267 (5.01), 249 (5.80), 247 (0.78), 237 (5.70), 236 (15.30), 234 (15.01), 232 (5.80), 227 (7.31), 226 (8.80), 225 (4.60), 212 (8.10), 211 (8.80), 210 (6.50), 203 (7.60), 202 (34.20), 201 (18.10), 199 (7.20), 198 (3.83), 187 (5.80), 186 (10.98), 185 (23.00), 184 (11.40), 179 (5.50), 178 (5.65), 168 (5.50), 167 (3.50), 166 (11.30), 162 (22.30), 161 (13.80), 160 (9.83), 147 (8.33), 146 (9.40), 145 (6.60), 135 (5.85), 134 (11.40), 132 (7.68), 122 (4.50), 121 (53.30), 120 (9.60), 118 (8.30), 113 (11.80), 112 (4.40), 107 (5.80), 106 (5.80), 105 (7.76), 98 (2.70), 97 (11.30), 96 (6.30), 92 (5.50), 91 (14.30), 89 (5.30), 79 (3.40), 78 (8.10), 77 (8.70), 65 (13.80), 64 (12.90), 63 (8.80), 57 (2.80), 56 (56.30), 55 (9.20), 51 (5.63). Anal. Found: C, 53.16; H, 4.22; N, 10.78; S, 8.27. C17 H17 N3 FeO2 S requires: C,
53.26; H, 4.44; N, 10.96; S, 8.35.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1613
ISSN 2229-5518
Compound 6a as pale brown crystals, yield 63%, m. p. 120oC. IR (KBr): 1720 – 1712 (br. C = O),
1628 (C = N), 1468 (C = S), 1605, 1585 (C = C) cm-1.1H–NMR (DMSO – d6 ): δ 2.18 (s, 3H, CO CH3 ), 2.34 (s, 3H, N = C–CH3 ), 4.16 – 4.47 (m, 9H, H – ferrocene ring), 6.68 – 7.81 (m, 6H, Ar–H and H-olefinic) ppm. 13C – NMR (DMSO – d6 ): δ 166.39, 158.33 (C = O), 167.20 (C = S), 146.31 (C = N), 133.24, 132.17, 130.89, 130.05, 129.53, 127.69, 125.19, 91.18 (C–aromatic and C–
olefinic), 84.84, 64.40, 69.80, 69.54, 69.47, 68.84, 68.38, 67.95, 67.42, 67.36 (C–ferrocene ring),
23.53, 23.30 (2 × CH ) ppm. MS: m/z (%) = 472(M+
+2, 6.30), 471(3.50), 470(M+, 9.20),
430(6.20), 429(7.70), 428(100), 427(13.20), 40495.30), 403(11.60), 228(1.30), 227(11.50),
226(11.80), 225(4.30), 212(7.20), 211(8.30), 210(5.50), 203(7.80), 202(31.20), 201(15.80),
199(5.40), 198(6.70),187(3.60), 186(8.10), 185(32.10), 184(11.30), 179(4.50), 178(6.30),
168(6.20), 167(4.20), 166(11.30), 162(19.20), 161(11.30), 160(6.70), 147(5.70), 146(6.60),
145(6.50), 135(17.10), 134(86.50), 133(11.20), 132(6.80), 122(4.30), 121(35.60), 120(6.90),
119(7.30), 113(11.20), 112(4.20), 107(6.30), 106(5.20), 105(7.20), 98(2.10), 97(11.20), 92(6.50),
91(17.20), 90(33.20), 89(16.20), 78(3.30), 77(45.20), 65(11.20), 64(9.80), 63(22.30), 57(11.20),
56(69.30), 55(8.20),51(11.20). Anal. Found: C, 61.11; H, 4.09; N, 8.78; S, 6.66. CR24RHR20RNR3RFeOR2RS
requires: C, 61.27; H, 4.25; N, 8.43; S, 6.81.
Compound 6b as pale brown crystals, yield 61%, m. p. 137oC. IR (KBr): 1722 – 1715 (br. C = O),
1632 (C = N), 1605, 1589 (C = C), 1463 (C = S), 1215, 1078 (C – O) cm-1.1H–NHR (DMSO–d ):
R6R
δ 2.21 (s, 3H, COCHR3R), 2.33 (s, 3H, N = C–CHR3R), 4.15 – 4.73 (m, 9H, H – ferrocene ring), 6.83 –
7.81 (m, 5H, Ar–H and H–olefinic) ppm. 13C–NMR (DMSO – d
): δ 168.20, 166.30 (C = O),
R6R
165.31 (C =S), 152.20 (C – O), 151.12 (C = N), 138.23, 136.62, 134.15, 130.26, 129.68, 128.71,
127.68, 120.61 (C –aromatic and C –olefinic), 83.80, 72.22, 70.51, 70.21, 70.15, 69.83, 69.72,
69.33, 68.11 (C – ferrocene ring), 23.18, 23.51 (2×CHR3R), 21.21(CHR3R) ppm. Anal. Found: C, 58.98;
4.02; N, 7.79; S, 6.02. CR26RHR22RNR3RFeOR3RS requires: C, 59.09; H, 4.17; N, 7.95; S, 6.06.
Compound 8 as pale brown crystals, yield 76%, m. p. 79 oC IR (KBr): 1708 (C = O), 1630 (C = N),
1605, 1589 (C = C), 1462 (C =S) cm-1.1H–NMR (DMSO – d
): δ 2.13 (s, 3H, COCH
), 2.37 (s,
R6R
R3R
3H, N = C–CHR3R), 4.12 – 4.79 (m, 9H, H – ferrocene), 7.25 – 7.88 (m, 6H, Ar–H and H–
imidazolidine) ppm. 13C – NMR (DMSO – d
): δ 180.39 (C = S), 168.89 (C = O), 149.22 (C = N),
R6R
134.74, 129.28, 129.06, 128.53, 128.24, 126.33, 126.13, 109.97 (C–aromatic and C –
imidazolidine), 80.04, 71.74, 70.38, 70.02, 69.24, 68.97, 68.58 (C – ferrocene), 22.87 (N = C-
+ + +
CHR3R), 18.06 (CO -CHR3R) ppm. MS: m/z (%) = 445(M
+2, 6.70), 444(M +1, 3.20), 443(M ,16.20),
403(6.50), 402(7.20), 401(100), 387(5.50), 386(9.50), 357(5.60), 228(11.60), 227(12.50),
226(21.20), 219(6.30), 218(18.30), 217(6.30), 212(5.40), 211(14.20), 202(4.70), 201(3.30),
197(6.10), 196(29.30), 195(16.50), 194(19.70), 193(17.20), 186(2.30), 185(40.10), 184(17.20),
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1614
ISSN 2229-5518
178(11.20), 177(15.30), 175(6.30), 161(6.20), 160(13.20), 159(6.20), 154(7.20), 153(22.30),
152(15.60), 151(5.90), 148(3.90), 147(4.20), 146(7.30), 138(3.10), 137(30.30), 136(19.70),
132(9.20), 131(7.50), 128(11.30), 127(6.30), 122(11.60), 121(52.20), 120(18.20), 119(6.70),
107(5.60), 106(4.90), 105(9.70), 104(6.20), 103(21.30), 102(12.30), 92(12.60), 91(21.20),
89(15.60), 89(17.60), 88(7.10), 78(9.90), 77(21.20), 76(17.30), 66(5.40), 65(17.20), 64(16.50),
63(32.30), 57(17.20), 56(67.20), 55(11.20), 54(11.20), 51(18.30). Anal. Found: C, 62.33; H, 4.57; N, 9.29; S, 7.07. C23 H21 N3 FeO4 S requires: C, 62.30; H, 4.74; N, 4.48; S, 7.22.
Centrifuged pellets of bacteria from a 24 h. old culture containing approximatelly 104 - 106
CFU (colony forming unit) per ml. were sepread on the surface of nutrient agar (typtone 1%, yeast extrcat 0.5%, NaCl 0.5%, agar 1%, 1000 ml. of distilled water, PH 7.00), which was autoclaved under 121oC for at least 20 min. Wells were created in medium with the help of a strile metallic bores and then cooled down to 45oC. The activity was determined by measuring the diameter of the inhibition zone (in mm.).100 µl of the tested samples (10 mg/ml and 50 mg/ml) were loaded into
the wells of the plates. All compounds were prepared in dimethylsulfoxid (DMSO), DMSO was loaded as control. The plates were kept for incubation at 37oC for 24 h and then the plates were examined for the formation of zone of inhibition. Each inhibition zone was measured three times by caliper to get an average value. The test was preformed three times for each bacteria culture. Ciprofloxacin was used as antibacterial standard drug.
Fungal strain was grown in 5 ml. sabourad dextrose broth (glucose: peptone; 40.10) for 3-4 days to achieve 105 CFU/ml cells. The fungal (culture co. 1 ml) was sepread out uniformly in the sabourad dextrose agar plates by sterilized triangular folded glass rod. Plates were left for 5-10 min. So that culture is properly adsorbed on that surface of sabourad dextrose agar plates. Now small wells of size (4 mm × 2 mm) were cut into the plates with the help of well culture and bottom of the wells were seald with 0.8% soft agar to prevent the flow of test sample at the bottom of the well.100
µl of the tested samples (10 mg/ml and 50 mg/ml) were loaded into the wells of the plates .All compounds were prepared in dimethylsulfoxide (DMSO), DMSO was loaded as control. The plates were kept for incubation at 30oC for 3-4 days and then the plates were examined for the formation of zone of inhibition. Each inhibition zone was measured three times by caliper to get an average value. The test was performed three times for each fungus. Flucomazole was used as antifungal
standard drug.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1615
ISSN 2229-5518
Human hepatocellular carcinoma (HepG-2) cells were obtained from the American Type Culture Collection (ATCC, Rock Villa, MD). The cells were grown on RPMI – 1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/ml gentamycin. The cells were maintained at 37oC in a humidified atmosphere with 5% CO2 and were subcultured two to three times a week.
The antitumor activity was evaluated on HepG-2 cell. The cells were grown as monolayer in growth RPMI – 1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/ml gentamycin. The monolayer of 10.000 cells adhered at the bottom of the wells in a 96-well microtiter plate incubated for 24 h at 37oC in a humidified incubator with 5% CO2 . The monolayers were then washed with sterile phosphate buffered saline (0.01M, PH 7.20) and simultaneously the cells were treated with 100 µl from different dilution of the test sample in fresh maintenance medium and incubated at 37oC. A control of untreated cells was made in the absence of the test sample. Six wells were used for each concentration of the test sample. Every 24 h the observation under the inverted microscope was made. The number of the surviving cells was determined by staining the cells with crystal violet followed by cell lysing using 33% glacial acetic acid and read the absorbance at 490 nm using ELISA reader (SunRise, TECAN, Inc, USA) after well mixing. The absorbance values from untreated cells were considered as 100% proliferation. The number of viable cells was determined using ELISA reader as previously mentioned before and the percentage of viability was calculated as [1 – (ODt /ODC)] 100%, were ODt is the mean optical density of wells treated with the test sample and ODC is the mean optical density of untreated cells. The 50% inhibitor concentration (IC50 ), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots.
We deeply appreciate the regional center for mycology and biotechnology Al-Zahar
University for helping on the evaluation of cytotoxicit-y against liver cancer cells and antimicrobial activity.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1616
ISSN 2229-5518
![]()
[1] (a) M. Meusel, M. Gutschow, Org. Prep. Proced. Int., 2004, 36, 391; (b) L. Chevolot, S. Padua, B. N. Ravi, P. C. Blyth and P. J. Scheuer, Heterocycles, 1977, 7, 891; (c) R. Fathiafshar and T. M. Allen, Can. J. Chem., 1988, 66, 45.
[2] (a) C. Jimenez, P. Crews, Tetrahedron Lett., 1994, 35, 1375; (b) I. S. Chen, C. T. Chang,W. S.
Sheen, C. M. Teng, I. L. Tsai, C. Y. Duh, F. N. Ko, Phytochemistry, 1996, 41, 525.
[3] C.-J. Fang, G. Han, Y.-J. Liu, C.-Y. Duan and Q.-J. Meng, Acta Cryst. (1999). C55, 2058-2060 [4] (a) A. Balog, M.E. Salvati, W.F. Shan, A. Mathur, L.W. Leith, D.D. Wei, R.M. Attar, J.P. Geng,
C. A. Rizzo, C. H. Wang, S. R. Krystek, J. S. Tokarski, J.T. Hunt, M. Gottardis, R. Weinmann, Bioorg. Med. Chem. Lett., 2004, 14, 6107 (b) X.Q. Zhang, G.F. Allan, T. Sbriscia, O. Linton, S.G. Lundeen and Z. H. Sui, Bioorg. Med. Chem. Lett., 2006, 16, 5763; (c) G.G. Muccioli, D. Martin, G.K.E. Scriba, W. Poppitz, J.H. Poupaert, J. Wouters, D.M. Lambert, J. Med. Chem.,
[5] T. Dylag, M. Zygmunt, D. maciag, J. handzlik, M. Bednarski, B. Filipek, K. Kiec-Kononowiez,
Eur. J. Med. Chem., 39, 1013 – 1027, (2004).
[6] N. Opacic, M. Barbaric, B. Zorc, M. Cetina, A. Nagl, D. Erkouic, M. Kradj, K. Pavelic, J.
Balzarini, G. Andrei, R. Snoeck, E. De Clerey, S. Raic-Malic, M. Mintas, Med. Chem., 48,
475-482, (2005).
[7] E. Latt Mann, W. O. Ayuko, D. Kinchinaton, C. A. Langley, H. Singh, L. Karimi, M. S. Trsdale,
,J.Phar.pharmacol , 55, 1259-1265, (2003).
[8] C. Carmi, A. Cavazzoni, V. Zuliani, A. Lodola, F. Bordi, P.V. Plazzi, R.R. Alfieri, P.G.
Petronini, M. Mor, Bioorg. Med. Chem. Lett., 16, 4021-4025, (2006).
[9] G. Singh, P.H. Driver, J.W. Sander, L. Sander, Brain, 128, 7-17, (2005).
[10] F. L. Wessels, T. J. Schwan and S. F. Pong, J. Pharm. Sci., 1980, 69, 1102.
[11] A.G. Caldwell, C.J. Harris, R. Stepney and N. Whittaker, J. Chem. Soc., Perkin Trans. 1,
[12] H.H. Merrit, T.J. Putnam, Arech. Neurol. Psychiatry, 39, 1003-1015, (1938). [13] M. Shiozaki, carbohydr. Res., 337, 2077-2088,(2002).
[14] J. Marton, J. Fnisz, S. Hosztafi, T. Timar, J. Agric. Food. Chem, 41, 148-152, (1993).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1617
ISSN 2229-5518
![]()
[15] (a) T.J. Kealy, P.L. Pauson, Nature 168 (1951) 1039-1040; (b) P.N. Kelly, A. Pretre, S. Devoy, I. O’Rielly, R. Devery, A. Goel, J.F. Gallagher, A. J. Lough, P.T.M. Kenny, J. Organomet. Chem. 692 (2007) 1327-1331; (c) M.F.R. Fouda, M.M. Abd-Elzaher, R.A. Abdelsamaia, A.A. Labib, Appl. Organomet. Chem. 21 (2007) 613-625.
[16] (a) H. Yu, L. Shao, J. Fang, J. Organomet. Chem. 692 (2007) 991-996; (b) M. Zora, M.
Görmen, J. Organomet. Chem. 692 (2007) 5026-5032. (c) M. Zora, O. Velioglu, J. Organomet. Chem. 693 (2008) 2159-2162.
[17] (a) B. Fabian, V. Kudar, A. Csampai, T. Zs. Nagy, P. Sohar, J. Organomet. Chem. 692 (2007)
5621-5632; (b) T. Mochida, F. Shimizu, H. Shimizu, K. Okazawa, F. Sato, D. Kuwahara, J. Organomet. Chem.692(2007)1834e1844. [28] B. Maity, M. Roy, A. R. Chakravarty, J. Organomet. Chem. 693 (2008) 1395-1399.
[18] (a) C. Biot, L. Delhaes, L.A. Maciejewaski, M. Mortuaire, D. Camus, D. Dive, J.S. Brocard, Eur. J. Med. Chem. 35 (2000) 707-714; (b) C. Biot, G.Glorin, L.A. Maciejewaski, J. S. Brocard, J. Med. Chem. 40 (1997) 3715-3718; (c) C. Biot, L. Delhaes, L.A. Maciejewaski, D. Camus, D. Dive, J.S. Brocard, Bioorg. Med. Chem. 7 (1999) 2843-2847.
[19] (a) I.M. El Deen, J.A. Hasanen, M. El Ashery, Inter. J. Innov. Res. In Science, Eng. And Technology, 3, 9702-9722, (2014); (b) H.K. Ibrahim, J.A. Hasanen, M.A. Zein and I.M. El Deen, Mens Agitat, 3, 54-72, (2008); (c) J.A. Hasanen, I.M. El-Deen, R.M. El-Desoky, A.M Abdalla, Res. Chem. Intermed. 2014, 40 (2) 537-553; (d) M.S. Refat, I.M. El-Deen, R.F.M. El- Shaarawy, Russ. J. Gen. Chem. 2014, 84 (3) 593-601.
[20] (a) H. K.Ibrahim, E. Eltamany, R.F.M.Elshaarawy, I.M. Eldeen, Maced. J. Chem. Chem.
Eng.2008, 27(1), 65-79; (b) R.F.M. Elshaarawy, C. Janiak, Eur. J. Med. Chem. 2014, 75, 31-
42; (c) R.F.M. Elshaarawy, Z.H. Kheiralla, A.A. Rushdy, C. Janiak, Inorg. Chim. Acta 2014,
421,110-122; (d) .F.M. Elshaarawy, C. Janiak, Tetrahedron, 2014, in press.
[21] C.-J. Fang, G. Han, Y.-J. Liu, C.-Y. Duan and Q.-J. Meng, Acta Cryst. (1999). C55, 2058-
2060.
[22] K.E. Cooper, A. Kavanagh, "Analytical microbiology",Vol-2 (Academic Press New York), 13, (1972).
[23] V. Betina, The chemistry and biology of antibiotres (Ed1) with nature RF Rekker,
Cechoslovakia (Elsevier Scientific Publishing, New York), (1983).
[24] T. Mosmann, "Rapid Colorimetric Assay for Cellular Growth and Survival: Application to
Proliferation and Cytotoricty Assays", J. Immunol. Methods, 65, 55-63, (1983).
[24] N. Gangadevi, J. Muthumary, "Preliminary Studies on Cytotoxic effect of Fungal taxol on
Cancer cell lines", African J/ Biotechnology, 6, 1382-1386, (2007).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 1618
ISSN 2229-5518
![]()
IJSER © 2015 http://www.ijser.org