International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
Expression of NT-3 and BDNF in cerebro-spinal fluid of patients with Tuberculous meningitis
Yun Li 1*, Chun Peng 2, Zi-Hua Zhang3, Xiao-Yan Zhang 1, Dan-Dan Li1, Jian Zhong 1
1Department of Neurology, the Affiliated Hospital of Dali University Dali 671000, Yunnan Province, China; 2Department of Technology, the Public Security Bureau of Dali Prefecture, Dali 671000, Yunnan Province, China; 3Department of ecsomatics, the Affiliated Hospital of Dali University. Dali 671000, Yunnan Province, China
*Corresponding Author: Yun Li, Doctor, associate chief physician,
Address: Department of Neurology, the Affiliated Hospital of Dali University, Dali 671000, Yunnan Province, China
Tel:86-872-2252646
Fax:86-872-2201168
E-mail: dlhliyun@163.com
Aim: To explore the expression of brain deriverd neurotrophic factors (BDNF) and neurotrophic factor-3 (NT-3) in the CSF of patients with Tuberculous meningitis (TBM). And elucidate the underlying mechanisms. Methodology: Prospective observational clinical study performed on 18 adults with TBM and 12 Adults with viral meningo-encephalitis (ME). 9 controls with non-inflammatory intracranial hypertention were also employed. Neurotrophic factor levels in the CSF were measured using an immunoenzymatic assay(ELISA). Results: Both NT-3 and BDNF protein level of CSF were higher in patients with TBM at acute period than the patients with viral ME (P<0.05). Whereas there was no statistical difference of BDNF protein level between the patients with TBM and non-inflammation intracranial hypertention group(P>0.05). The patients with viral ME also showed no statistical differnce of BDNF protein level compared with non-inflammation intracranial hypertention group(P>0.05). NT-3 expression was higher in the patients with TBM than non-inflammation intracranial hypertention group(P<0.05). But the patients with viral ME showed no significant difference compared with non-inflammation intracranial hypertention group(P>0.05). Furthermore, NT-3 protein level in the CSF of patients with TBM at acute period was higher than BDNF protein level (P<0.05). Conclusion: The expression variations of NT-3 and BDNF protein level in CSF may reflect an endogenous attempt at neuroprotection against biochemical and molecular changes during both TBM and viral ME at the acute period. Which suggested a marked and rapidly activated intracerebral neurotrophic factors biosynthesis after the patients subjected to TBM. The expression of NT-3 and BDNF is likely to play a
neuro-immunomodulatory role in the process of TBM.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
Tuberculous meningitis (TBM) is the most severe form of extrapulmonary tuberculosis and causes high mortality and a distressing level of neurological morbidity. Even with standard anti-tuberculous therapy short-term mortality is high;
ranging from 20–69% [1,2,3.4]. TBM a medical emergency, is still a major cause of serious illness in many parts of the world. It would not be out of place to remind ourselves that fundamental questions regarding the pathogenesis, diagnosis, treatment, and management of CNS tuberculosis remain unanswered despite availability of effective chemotherapy. In the current, the emergence of multidrug-resistant strains poses a serious threat to the control of this pathogen. So TBM remains a serious public-health problem. However, the challenge to adequately diagnose and deal with its morbidity has yet to be substantially met. The best and most rapid method of diagnosis, optimal treatment drugs and duration of therapy are questions which have yet to be adequately addressed[5,6,7]. It has been shown that CD4+ T-lymphocytes are most important in the protective response against Mycobacterium tuberculosis . A study of immunological parameters showed a relation between the development of tuberculous meningitis (TBM) and significantly lower count of CD4 T-lymphocytes when compared with others who had pulmonary complex only[8].Which highlights the importance of cellular immunity, conducted by T-lymphocytes, in the outcome of tuberculosis. Development of an effective vaccine against tuberculosis
hinges on an improved understanding of the human immune response to Mycobacterium tuberculosis (Mtb). The emergence of drug resistant tuberculosis poses a serious threat to the control of this pathogen, and the development of drugs that are active against the resistant strains is vital[9]. So more and more researchers have begun to explore the immune therapy of TBM.
Neurotrophic factors (NTF), consists of a family of polypeptides,such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotropin 3 (NT-3), neurotrophin 4 (NT-4), epidermal growth factor (EGF), fibroblast growth factor (FGF), glia-derived growth factor (GDNF), vascular endothelial growth factor (VEGF), insulin growth factor
(IGF),as well as their high-affinity receptors TrkA, TrkB and TrkC. Every kinds of neurotrophins play an important
physiological role by binding to corresponding receptors. All neurotrophins mediate their effects via activation of one or more Trk receptors. Nerve growth factor together with possibly other neurotrophins such as BDNF, NT3 , NT4/5or GDNF are important modulators of immunity[10,11,12,13,14]. Neurotrophic factors play an important role in neuronal survival and recovery after acute injury to the central nervous system (CNS) and may represent an additional therapeutic target for treatment of viral encephalitis [15]. It is expected that NTF may be the underlying factors responsible for the
neuromodulatory of immunologic process of TBM.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
In the family members of neurotrophins , BDNF and NT-3 are of particular interest in this regard. As BDNF, since its receptor (trkB) is expressed in a broad range of neurons throughout the brain [16]. Whereas, NT-3 is another very important neurotrophin, which mediate their effects via activation predominantly the TrkC receptor but can also bind to other Trk receptors [17]. All of these are prerequisite for a beneficial effect in diseases affecting multiple regions of the brain as is the case in TBM. The present study, therefore, sought to test whether the expression of BDNF and NT-3 have a correlation with the occurrence of TBM.
Study population and the collection of cerebrospinal fluid
We conducted a prospective observational clinical study among adults patients admitted from June 2011 to April 2012 with diagnosis of TBM and viral meningo-encephalitis(ME) to the neurology department of the affiliated hospital, DaLi university. All the patients were grouped according to aetiology, findings on head CT scan, clinical and laboratory characteristics. TBM Standardised clinical case definition accroding to the international tuberculous meningitis workshop took place in Cape Town, South Africa, in May, 2009[18]. Patients then move up or down the diagnostic pyramid as subsequent results become available and are classified into definite, probable, possible, or not tuberculous meningitis according to diagnostic criteria. Definite tuberculous meningitis: microbiological identification or evidence from commercial nucleic acid amplification tests of central neverous system Mycobacterium tuberculosis infection. Probable tuberculous meningitis: when imaging is available a diagnostic score of 12 or above is required, and when imaging is not available, a diagnostic score of 10 or above is required. Possible tuberculous meningitis: when imaging is available a diagnostic score of 6–11 is required, and when imaging is not available, a score of 6–9 is required. As controls, we used CSF collected from patients with non-inflammatory intracranial hypertention who had undergone benign intracranial hypertention. Controls were matched for age and sex, respectively. All patients were subjected to hematological determinations, analysis of urine, electrocardiography, chest radiography, and carotid ultrasound at admission. SIEMENS sensation 64-slice spiral CT and PHILIPS 1.5T MR imaging machine were used for the imaging assessment. Cranial CT scans of all patients were finished within 24 h after admission, and cranial MR scans of all patients were completed within
72 h after admission. To measure the levels of neurotrophic factors, we collected samples of CSF using lumbar puncture. All samples were obtained in the acute phase of the illness, from the first lumbar puncture, before any treatment or at the benginning of 3 days. The CSF samples were submitted for microbiological and biochemical analysis (germ and mycete culture , cellularity and glucose-protein concentration in the CSF). Detection of acid-fast bacilli (AFB) and culture isolation of Mycobacterium tuberculosis from cerebrospinal fluid (CSF) were also performed. The CSF was centrifuged at 1000
r/min for five minutes to remove the precipitate and snap-frozen and the supernatant was immediately stored at -80°C until
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
analysis. The study was approved by the Institutional Ethical Review Board of Clinic Medical College of DaLi University, and written informed consents were obtained from all patients and/or relatives and the control subjects.
Neurortophic factor assays
BDNF The endogenous BDNF levels were determined in triplicate in undiluted CSF by commercially available sand-wich-type enzyme-linked immunosorbent assays(Chemikine, Millipore Corporation, USA) following the manufacturer's instructions. To detail, Polystyrene 96-well microtube immunoplates (Nunc) were place the desired number of ChemiKine Brain Derived Neurotrophic Factor strips. Add 100 µL of Standards 0 through 7 or samples to wells. It is recommended that standards and samples be run in duplicate. Seal the plate with a plate sealer. Incubate the plate at 2-8°C overnight (on a shaker if possible). Gently remove the plate sealer and wash the plate at least 4 times. Using the multichannel pipet add 250µL of Wash Buffer to each well; flick and blot the plate. Repeat this procedure for a total of 4 times. Add 100µL of the diluted biotinylated mouse anti-BDNF monoclonal antibody (dilute the biotinylated antibody
1:1,000 with Sample Diluent) to each well. Cover the plate and incubate at room temperature for 2-3 hours Wash the plate. Add 100µL of the diluted streptavidin-HRP conjugate solution (dilute the HRP conjugate 1:1,000 with Sample Diluent) to each well. Cover the plate and incubate at room temperature for 1 hour. Wash the plate 4 times. Warm TMB to room temperature. Add 100 µL of TMB/E Substrate to each well. Incubate at room temperature for 15 minutes. Stop the reaction by adding 100 µL of Stop Solution to each well. Optical density was measured at 450 nm, using an ELISA reader (Dynatech), and the values of standards and samples were corrected by taking non-specific binding into consideration. BDNFconcentrations were determined from the regression line for the BDNF standard. The sensitivity was 7.8 pg/ml for
BDNF. And there was no significant cross-reactivity with other molecules of the NGF family (i.e, NGF,NT-3 and NT-4/5).
BDNF concentration was expressed as pg/ml for liquid samples. All assays were performed in duplicate.
NT-3 NT-3 concentration were measured by a highly sensitive two-site immunoenzymatic assay kit(Chemikine, Millipore Corporation, USA), As performed for the BDNF assay following the manufacturer's instructions. With the ChemiKine assay system, sheep polyclonal antibodies generated against human NT-3 are coated onto a microplate and are used to capture
NT-3 from a sample. NT-3 specific, biotin conjugated, mouse monoclonal antibodies detect the captured NT-3. After addition of streptavidin-enzyme, substrate and stop solution the amount of NT-3 is determined. Optical density was also measured at 450 nm, using an ELISA reader (Dynatech), and the values of standards and samples were corrected by taking non-specific binding into consideration. NT-3 levels were determined from the regression line for the NT-3 standard. The
sensitivity was 30pg/ml for NT-3. And there was no significant cross-reactivity with other molecules of the NGF family (i.e.,
NGF, BDNF and NT-4/5). NT-3 concentration was expressed as pg/ml for liquid samples. All assays were performed in
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
duplicate.
After the collection of primary data, further data collection and analysis were done by observers blind to the experimental manupulations. When the last average data were available, they were analyzed by the SPSS 13.0 statistical
analysis software package. Data were presented as means ± standard deviation of the mean (mean±SD). Statistical
differences among groups were determined by One-Way ANOVA. Different variance in the same group comparisons were made by two sample t-test . P<0.05 represents significant difference.
40 patients with tuberculous meningitis, viral meningoencephalitis and benign intracranial hypertention were admitted to this study. The patients were aged 18 y to 64 y, with a mean age of 38.6 ±4.28 y (median age: 25.3 y). Table 1 shows clinical and demographic characteristics of these samples. The patients were divided into three experimental groups as shown in Table1. Group I patients were subjected to TBM; Group II patients were subjected to viral ME; Group III were control patients consisted of non-inflammation benign intracranial hypertention.
Table1 Patients grouping according to clinical and etiological characteristics![]()
![]()
Grouping | I | II | III | |
etiology | Tuberculous meningitis | Viral ME | benign intracranial hypertention | |
Number (n) | 18 | 13 | 9 | |
Average age(years) | 34 | 25 | 31.4 | |
Gender(n) Femal Male | 5 13 | 8 5 | 4 5 |
![]()
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
BDNF level among different group patients
All patients admitted to this study were examined BDNF protein level in CSF by ELISA method. The result showed that BDNF protein level was significantly higher in patients with TBM at acute period than the patients with viral ME (P<0.05). And there was no statistical difference between the patients with TBM and non-inflammation control group(P>0.05). The patients with viral ME also showed no difference compared with non-inflammation control group(P>0.05).(Fig 1, Table 2)![]()
![]()
![]()
![]()
![]()
Table 2 BDNF and NT-3 levels in CSF of patients in different group ( x ±s)(pg/ml)
Grouping | Number of patients | BDNF level( x ±s) | NT-3 level ( x ±s) |
I | 18 | 928.18±247.55* | 1185.29±237.06*Δ |
II | 13 | 618.08±128.63 | 882.90±171.14 |
III | 9 | 763.67±185.81 | 763.67±141.41 |
![]()
Note: *p <0.05: group I compared with group II; Δp <0.05: group I compared with group III.
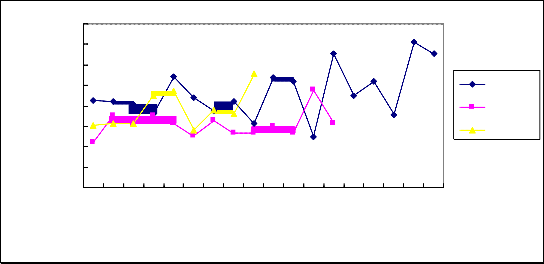
Figure.1 BDNF protein level in cerebro-spinal fluid of patients with TBM and viral ME compared with control group. The number of patients are indicated by the horizontal lines, Cerebro-spinal fluid BDNF concentration are indicated by the vertical lines. TBM:Tuberculous meningitis; V-ME:Viral meningo-encephalitis; Control: non-inflammatory benign intracranial hypertention
NT-3 level among difference group patients
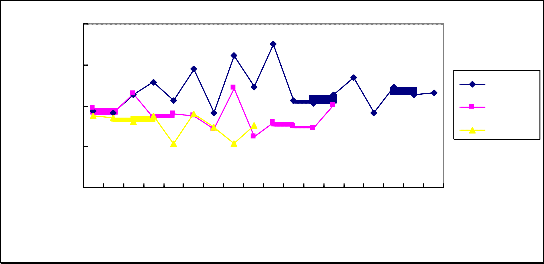
The result showed that NT-3 level in CSF was the highest in patients with TBM at acute period compared with the patients with viral ME and non-inflammation control group (P<0.05). But the patients with viral ME showed no statistical difference compared with non-inflammation control group(P>0.05).(Fig 2, Table 2).
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
Figure.2 NT-3 protein level in cerebro-spinal fluid of patients with TBM and viral ME compared with control group. The number of patients are indicated by the horizontal lines, Cerebro-spinal fluid BDNF concentration are indicated by the vertical lines. TBM:Tuberculous meningitis; V-ME:Viral meningo-encephalitis; Control: non-inflammatory benign intracranial hypertention.
The expression of BDNF and NT-3 in the patients with TBM
The results of ELISA assays suggested NT-3 level of CSF in patients with TBM at acute period was higher than
BDNF(P<0.05) (Fig 3, Table 3).![]()
![]()
![]()
![]()
Table 3 BDNF and NT-3 levels in CSF of patients with TBM. ( x ±s)(pg/ml)
Neurotrophic factor | Numbers of the Sample | Protein level ( x ±s) |
BDNF | 18 | 928.18±247.55 |
NT-3 | 18 | 1185.29±237.06* |
![]()
Note: *p <0.05, NT-3 level compared with BDNF level in CSF of patients with TBM.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
3000
2500
2000
1500
1000
500
0
1 3 5 7 9 11 13 15 17
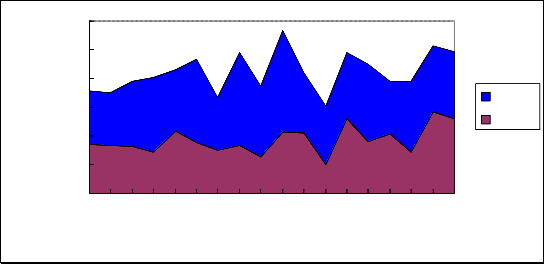
Figure. 3 The expression of BDNF and NT-3 level in cerebro-spinal fluid of patients with TBM. The number of patients are indicated by the horizontal lines, Cerebro-spinal fluid BDNF and NT-3 concentration are indicated by the vertical lines. NT-3: NT-3 level in CSF of patient with TBM; BDNF: BDNF level in CSF of patient with TBM.
The present study investigated first the expression of neurotrophin-3 and BDNF in cerebro- spinal fluid of the patients with TBM. Despite the limited patient sample so far evaluated, the present study provides evidence that NT-3 and BDNF expression in the CSF of the patient with TBM and viral ME is different, suggested that they serve different functions in CNS infections.
Inflammatory processes in the central nervous system (CNS) are considered neurotoxic, although recent studies suggest that they also can be beneficial and confer neuroprotection [19,20]. Cells from the immune system have been detected in CNS injury and found to produce and secrete a variety of neurotrophins such as NGF, BDNF, NT-3 and NT-4/5, and to express (similarly to neuronal cells) members of the tyrosine kinase (Trk) receptor family such as TrkA, TrkB and TrkC [21] . Tuberculous meningitis (TBM) is the most devastating form of tuberculosis. Both intracerebral and peripheral blood immune responses may be relevant to pathogenesis, diagnosis, and outcome[22]. Immune-driven inflammation in the confines of the subarachnoid space has long been considered as central to the cerebral pathology and outcome from TBM.
In agree with above study, the present study proved neurotrophins level increased in CSF occurred early in the patient with TBM. And the elavated degree of the neurotrophins in the patients with TBM have statistical difference compared with viral ME(P<0.05). It has reported the T4+/T8+ ratio of peripheral blood and CSF reduced in cases of tuberculous meningitis, and such changes were also found in peripheral blood of viral encephalitis patients [23]. Most previous studies reported the
expression of neurotrophic factors ( NGF, BDNF, NT-3 and so on) in cerebrospinal fluid and/or plasma of patients or animal
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
model with viral and or bacterial meningoencephalitis[24,25,26,27,28]. To the best of our knowledge, our study is the first port investigating the expression of neurotrophins in CSF of patients with TBM. Importantly,our study has demonstrated the immunoregulatory dysfunctions presenting in these neurological disorders were different between tuberculous meningitis and viral ME. Both BDNF and NT-3 protein level were significantly higher in the patients subjected to TBM than viral ME.
The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 (NT-3) and NT-4 play a pivotal role in the development of the nervous system [29]. The nervous and immune systems employ overlapping mechanisms and shared mediators that promote cross-talk between the two systems[30].Previous document reviewed the increased production of NGF and other trophic factors in central nervous system (CNS) during these diseases can suppress inflammation by switching the immune response to an anti-inflammatory, suppressive mode in a brain-specific environment. trophic factors networks in the adult CNS not only protects axons and myelin but appear to also actively contribute to the maintenance of the brain immune privilege [31]. A large body of work supports the proposal that there is a significant correlation between neurotrophins and central nervous system infection[24,25,26,27,28]. The present study noted a significant increase in NT-3 and BDNF CSF levels for the patient with TBM. Most intriguingly, our findings provide new insight into BDNF and NT-3 CSF levels were markedly elevated in the patient with TBM in the acute phase compared with viral ME. The results of our study also showed that the expression of NT-3 was higher than BDNF in the patients in TBM group.To our knowledge, these data are the first to completely demonstrate the expression of neurotrophins in the CSF for the patients with TBM. These findings should provide another avenue for the pathogenesis and treatment for TBM. They could also provide some basis for further clinic trial.
The expression variations of NT-3 and BDNF protein level in CSF may reflect an endogenous attempt at neuroprotection against biochemical and molecular changes for the patients with TBM. Which probably play a neuro-immunomodulatory role in the process of TBM.
1.Padayatchi N, Bamber S, Dawood H, et al Multidrug-resistant Tuberculous meningitis in children in Durban, South Africa. Pediatr Infect Dis J .2006;25: 147–50.
2 Porkert MT, Sotir M, Parrott-Moore P, et al. Tuberculous meningitis at a large inner-city medical center. Am J Med Sci
1997;313: 325–31.
3 Karstaedt AS, Valtchanova S, Barriere R, et al. Tuberculous meningitis in South African urban adults. QJM 1998;91:
743–7.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
4 Christensen ASH, Andersen AB, Thomsen VO, et al. Tuberculous meningitis in Denmark: a review of 50 cases. BMC Infect Dis .2011 ;11: 47.
5 Javaud N, Certal Rda S, Stirnemann J,et al. Tuberculous cerebral vasculitis: retrospective study of 10 cases. Eur J Intern
Med. 2011;22(6):e99-104.
6 Bryan-Rock R, Olin M, Baker CA, et al. Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin
Microbiol Rev. 2008;21(2):243–61.
7 Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol 2005; 4(3): 160–70.
8 Davoudi S,Rasoolinegad M, Younesian M,et al.CD4+ Cell Counts in Patients with Different Clinical Manifestations of
Tuberculosis.Davoudi S, Braz J Infect Dis. 2008;12(6):483-6.
9 Galimi R. Extrapulmonary tuberculosis: tuberculous meningitis new developments. Eur Rev Med Pharmacol Sci.
2011;15(4):365-86.
10 Stanisz AM, Stanisz JA. Nerve growth factor and neuroimmune interactions in inflammatory diseases. Ann N Y Acad
Sci. 2000;917:268-72.
11 Scuri M, Samsell L, Piedimonte G.The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets.
2010;9(3):173-80.
12 Nockher WA, Renz H.Neurotrophins in inflammatory lung diseases: modulators of cell differentiation and neuroimmune interactions. Cytokine Growth Factor Rev. 2003;14(6):559-78
13 Lommatzsch M, Braun A, Renz H.Neurotrophins in allergic airway dysfunction: what the mouse model is teaching us. Ann N Y Acad Sci. 2003;992:241-9.
14 Skaper SD.Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol.
2001;24(1-3):183-99.
15 Sellner J, Lenhard T, Haas J, et al. Differential mRNA expression of neurotrophic factors GDNF, BDNF, and NT-3 in experimental herpes simplex virus encephalitis. Brain Res Mol Brain Res. 2005;137(1-2):267-71
16 Bifrare YD, Kummer J, Joss P, et al. Brain-Derived Neurotrophic Factor Protects against Multiple Forms of Brain Injury in Bacterial Meningitis. The Journal of Infectious Diseases 2005; 191(1):40–5
17 Bartkowska K, Turlejski K, Djavadian RL.. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp 2010, 70(4): 454–467.
18 Marais S,Thwaites G, Schoeman JF,et al. Tuberculous meningitis: a uniform case definition for use in clinical
research.Lancet infection Dis.2010,10(11):803-812.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012
ISSN 2229-5518
![]()
19 Pajoohesh-Ganji A, Knoblach SM, Faden AI,et al. Characterization of inflammatory gene expression and galectin-3 function after spinal cord injury in mice. Brain Res. 2012 Aug
20 Gu JH, Ge JB, Li M,et al. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012 Jul 28.
21 Tabakman R, Lecht S, Sephanova S,et al. Interactions between the cells of the immune and nervous system:
neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res. 2004;146:387-401
22 Simmons CP, Thwaites GE, Quyen NT,et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176(3):2007-14.
23 Cao L. Preliminary studies of T lymphocyte subsets in patients with neurologic diseases. Zhonghua Shen Jing Jing Shen
Ke Za Zhi. 1990 ;23(3):159-61.
24 Kizawa-Ueda M, Ueda A, Kawamura N,et al. Neurotrophin levels in cerebrospinal fluid of adult patients with meningitis and encephalitis. Eur Neurol. 2011;65(3):138-43.
25 Chiaretti A, Antonelli A, Piastra M, et al. Expression of neurotrophic factors in cerebrospinal fluid and plasma of children with viral and bacterial meningoencephalitis. Acta Paediatr. 2004;93(9):1178-84.
26 Soontornniyomkij V, Wang G, Pittman CA,et al. Expression of brain-derived neurotrophic factor protein in activated microglia of human immunodeficiency virus type 1 encephalitis. Neuropathol Appl Neurobiol. 1998;24(6):453-60.
27 Li L, Shui QX, Liang K,et al. Brain-derived neurotrophic factor rescues neurons from bacterial meningitis. Pediatr
Neurol. 2007;36(5):324-9.
28 Tauber SC, Stadelmann C, Spreer A,et al. Increased expression of BDNF and proliferation of dentate granule cells after bacterial meningitis. J Neuropathol Exp Neurol. 2005;64(9):806-15.
29 Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res.
2004;146:347-67.
30 Hohlfeld R,Kerschensteiner M, Meinl E. Dual role of inflammation in CNS disease. Neurology. 2007;68(22 Suppl
3):S58-63
31 Villoslada P, Genain CP, Role of nerve growth factor and other trophic factors in brain inflammation. Prog Brain Res.
2004;146:403-14.
IJSER © 2012 http://www.ijser.org