International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 479
ISSN 2229‐5518
Ethanol Synthesis Using Yeast Anchored on Calcium Alginate and Clay Support. Lilian Amen Osawemwenze, Gideon Majiyebo Adogbo
Abstract- Glucose fermentation was investigated using yeast (saccharomyces cerevisiae) as free cells immobilized on calcium alginate and clay supports to compare their ethanol yields. The substrate concentrations of 312, 260, 208, 156 and 104g/L were fed into batch and fedbatch fermenters on both supports. Immobilization of yeast cells using clay support gave higher ethanol product yield in both batch and fedbatch contact processes compared to the calcium alginate support. The fedbatch process gave higher ethanol yield and efficiency compared to the batch process as a result of substrate inhibition and catabolite repression in the batch process. At substrate concentration of 208g/L, the ethanol yield appeared to be independent of the feed contacting pattern for both supports.
Key words: Immobilization, Sodium Alginate, Calcium Chloride, Gel entrapment, Physical adsorption, Clay.
—————————— ——————————
1 INTRODUCTION
thanol can be produced by fermentation of sugars from agricultural products or waste plant materials; whichever substrate is chosen, attention must be paid to the overall economics and energy consumption [1]. Ethanol has become a viable source of unconventional fuel thus technologies are being improved on to increase the volumetric productivity of ethanol. The use of immobilized whole cells in industrial processes has attracted considerable attention during the past few years due to advantages over traditional processes. Shuvashish [2] has shown that Immobilization provides high cell concentrations and cell reuse, it eliminates cell washout and the
costly processes of cell recovery and cell recycle. Self‐aggregating yeast strains, their entrapment in an organic matrix or adsorption on organic or inorganic supports, are the most common methods for cells immobilization [3]. Immobilization using calcium alginate beads is a form gel entrapment, alginate is an anionic polymer which has the ability to form a gel when it comes in contact with bivalent cations such as Ca2+.
————————————————
Lilian Amen Osawemwenze is a graduate of chemical Engineering
Department, Ahmadu Bello University, Nigeria.
Email: liamenosas@yahoo.com
Gideon Majiyebo Adogbo is currently a Doctor of Chemical
Engineering in Ahmadu Bello University, Nigeria.
Email: adogbogm@yahoo.com
[4], [5], [1], [2] and [6] carried out works using calcium alginate bead as a support.
Although bead disruption risk is high in the
presence of substances capable of chelating calcium, it has been shown that particle drying or other agents which promote shrinking increases mechanical stability of the alginate beads [7]. The use of clay is a form of physical adsorption. Aikaterini [8] carried out a work on the effective immobilization of candida antartica using clay modified with octadecyl trimethyl ammonium surfactant. Yeasts particularly Saccharomyces spp., are usually the choice for industrial ethanol production, because of their good fermentative capacity, high tolerance to ethanol and other inhibitors and the capacity to grow rapidly under the anaerobic conditions at high osmotic activities and ethanol concentration [9]. This work is aimed at producing ethanol from sucrose with five varying concentrations using saccharomyces cerevisiae cells (yeast) as catalyst immobilized on different supports of calcium alginate bead and clay. The ethanol production in the supports using batch and fedbatch process would be compared.
2 MATERIALS AND METHODS
2.1 Feed Medium
Laboratory sucrose was prepared in varying concentrations for various runs for the batch and fedbatch process with distilled water. 0.4g of
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 480
ISSN 2229‐5518
Ammonium Sulphate, 0.3g of Magnesium Sulphate
Pentahydrate and 0.5g of Potassium Dihydrogen Orthophosphate to serve as nutrient for the yeast cells were added and mixed thoroughly. Sulphuric acid was used to adjust the pH of the solution to
5.0. The medium was sterilized at a temperature of
112°c for fifteen minutes; it was then cooled to
30°c.
2.2 Immobilization of Yeast Cell in Calcium alginate
0.9g of sodium alginate was dissolved in 100ml of water which was sterilized for about fifteen
minutes at a temperature of 112°c, it was cooled to a temperature of 30°c after which dry yeast of 2.5g was dissolved in the solution. The cell alginate mixture was poured into a sterilized hypodermic needle and was fed drop wise into a flask containing sterilized CaCl2 solution. The droplets instantaneously reacted with CaCl2 which precipitated a gel form calcium alginate enzyme complex of spherical beads. The beads were kept in the solution for about fifteen minutes; the supernatant fluid was removed after which the beads were washed in distilled water to remove any yeast cell attached to surface of the bead. The beads were then dried. The mass of the dry beads was weighed; the alginate beads and substrate were introduced into the fermenter.
This operation was carried out for both the batch processes and the fedbatch process.
2.3 Immobilization of Yeast Cell in Clay.
A sieve of 0.5mm aperture was used to obtain the clay particles after which it was thoroughly washed with distilled water. The clay was placed in an oven at 180°c for fifteen minutes to get it sterilized. 20ml of Yeast culture was anchored on
20g of clay for two hours, allowing the cells to mix
with the clay and to get attached in the pores of the clay. The supernatant liquid was drained out after which the immobilized clay and substrate were introduced into the fermenter from the top through the opening using a glass funnel. This operation was carried out for both the batch and fedbatch process.
2.4 Product analysis
The amount of ethanol produced was analyzed using a digital spectrophotometer at a wavelength of 575nm to get its absorbance. Known
concentrations of ethanol were also analyzed to
get their corresponding absorbance which was used as a standard to get the concentrations of the ethanol product.
The unconverted sucrose was also analyzed using
a spectrophotometer at a wavelength of 600nm to get its absorbance. Known concentrations of sucrose were also analyzed to get their corresponding absorbance which was used as a standard to get the concentrations of the unconverted sucrose.
3. RESULTS AND DISCUSSION

In determining the efficiency and yield of the processes, the following equations were used:
. (1)
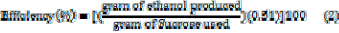
In terms of weight, every gram of glucose can theoretically yield 0.51g of ethanol. It is assumed that 50% of glucose was used to produce ethanol and 50% of it to produce CO2; thus there is a weight decrease due to the amount of CO2 removed from the system and the amount of ethanol that was produced [10] hence,
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 481
ISSN 2229‐5518
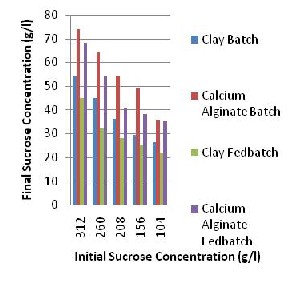

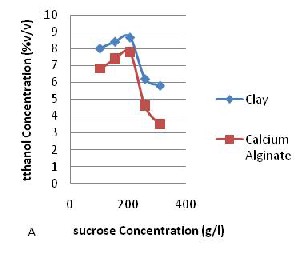
Fig. 1: Ethanol Production at Different Sucrose
Concentration for (A) Batch Process (B) Fedbatch.
In Fig.1, with increase in concentration in the fedbatch process from 104g/l to 312g/l ethanol production increases. The yeast cells used almost all the substrate given to the system during fermentation. This is an expected result since substrate inhibition and catabolite repression were prevented by intermittent feeding of the substrate. Intermittent addition of sucrose improves the efficiency of the fermentation by maintaining a low substrate concentration. Optimization of environmental conditions such as growth, production phase and the age of the culture is also possible in the fed batch mode of operation. However, expensive instruments such as process computers are required in large plants to keep the substrate concentration constant, this is a major disadvantage. From Fig. 1 above, the clay gave more ethanol yield compared to the calcium alginate.
In the batch process, with increase in concentration from 104g/l to 208g/l ethanol production increased thus the problem of limited substrate did not occur but as the concentration increased from 260g/l to
312g/l, ethanol production decreases as a result of substrate inhibition and catabolite repression; this shows that with increase in concentration from
260g/l there is cell growth, the yeast cells reproduce instead of producing ethanol as a by‐ product.
Fig. 2: Unconsumed Sucrose Concentration.
The Fig. 2 above shows the amount of unused sucrose for ethanol production. The batch process has higher amount of unused sucrose for both the clay and calcium alginate support. This result is in line with that carried out by Gough et al., [11] where the detrimental effect of high sugar concentration was studied in kluyeromyces marxians and a sucrose concentration of more than
23% in molasses was found to affect ethanol production [12]. The results of fermentation in which clay is used as a support are high when compared to calcium alginate beads from Fig. 1A, the reasons for this may include the loss of activity during immobilization, high intracellular ethanol concentration or diffusion limitations between the calcium alginate surface and yeast cell; according to Tartaridis et al., [7] and Panos et al., [6] beads disruption risk is high in the presence of substance like phosphate, lactate capable of chelating calcium and can cause destabilization of the gel. In the clay support no turbidity was observed during fermentation this shows that the yeast cells did not escape from the support to produce ethanol in free form indicating that the immobilization process using cell adsorption technique was efficient; this conforms to the work carried out by Aikaterini et al., [8] where candida antarctica was immobilized in organic modified clay and the immobilized enzyme retained a significant part of its activity after repeated use.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 482
ISSN 2229‐5518
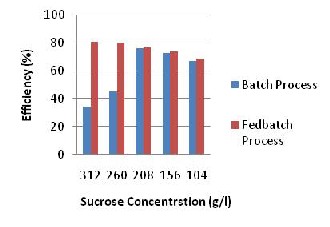

Fig. 3: Ethanol Concentration Using Varying
Sucrose Concentration.
From the Fig.3 above, there is a point of intersection at 208g/l between the clay batch and clay fedbatch indicating that at 208g/ l the amount of ethanol produced is the same. Also, from a concentration of 104g/l to 208g/l, there is no significant difference in the amount of ethanol produced; since batch process is easier to use and does not need much process control it can be applied for sucrose concentration of 104g/l to
208g/l in producing ethanol to reduce cost but beyond 208g/l, there is a large difference in the amount of ethanol produced in the clay batch and clay fedbatch; thus for sucrose concentration greater than 208g/l the fedbatch process should be used in producing ethanol. This also applies to the calcium alginate batch and calcium alginate fedbatch. In essence when producing ethanol with a sucrose concentration of 104g/l to 208g/l, the batch process can be used but beyond 208g/l the fedbatch process should be employed to save cost and for efficiency in production.
.
Fig. 4: Efficiency Histogram for the Batch and
Fedbatch Process using calcium alginate support.
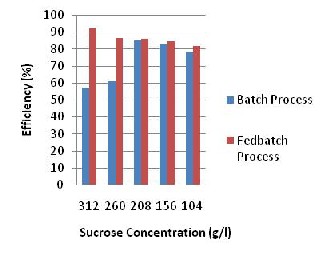
Fig. 5: Efficiency Histogram for the Batch and
Fedbatch Process using clay support.
Comparing the efficiency histogram of the batch and fed batch process of the Calcium Alginate support in Fig. 4 and Clay in Fig. 5, it shows that the fed batch process has higher efficiencies at different concentrations compared to the batch process but significant difference is seen in 260g/l and 312g/l.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 483
ISSN 2229‐5518
In the immobilization process it is speculated that the matrix may concentrate nutrient at the liquid solid interface thus allowing dense cell population to develop [13]. There is also the possibility that an immobilized cell grows better than a free cell due to limited motion it experiences even though there is no existing data to demonstrate this.
CONCLUSION.
Immobilization by physical adsorption using clay gave higher yield compared to gel entrapment using calcium alginate. The amount of ethanol produced was 3.5, 4.6, 7.78, 7.4 and 6.8% (v/v) in
the batch process, 8.2, 8.0, 7.8, 7.5 and 7.0% (v/v) in the fedbatch process for 312, 260, 208, 156
and 104g/L of sucrose concentration respectively
using calcium alginate support. Clay yielded
5.8, 6.2, 8.65, 8.4 and 8.0 % (v/v) of ethanol in the
batch process, 9.4, 8.8, 8.76, 8.59 and 8.3%
of ethanol in the fedbatch process for 312, 260, 208,
156 and 104g/L of sucrose concentration
respectively. The fedbatch process gave higher ethanol yield and efficiency compared to the batch process as a result of substrate inhibition and catabolite repression in the batch process which was counteracted in the fed batch process by intermittent feeding of the substrate. As the
cells grew and were confined to a limited space they had limited movement hence the consumption of sucrose was faster and ethanol yield was rapid. When producing ethanol with a sucrose concentration of 104g/L to 208g/L, the batch process can be used but beyond 208g/L the fedbatch process should be employed to save cost and for efficiency in production. At substrate concentration of 208g/L, the ethanol yield appeared to be independent of the feed contacting pattern for both supports.
REFERENCES
[1] R. Marica, M. Ljiljana, N. Svetlana, V. Maja and N. Viktor, “Bioethanol production by immobilized Sacharomycescerevisiae var. ellipsoideus cells” African journal of biotechnology , Vol. 8, No.3, pp464‐471, 2009.
[2] B. Shuvashi,C. Ramesh ,C.M Rama ,
“Comparative study of bioethanol production from mahula (Madhuca latifolia L.) Flowers by immobilized cells in Calcium Alginate beads” Journal of scientific and industrial Research, Vol. 69, pp 427‐475, 2010.
[3] C.M.S.G Baptista, J.M.A Cóias, A.C.M Oliveira, N.M.C Oliveira, J.M.S Rocha, M.J Dempsey, K.C Lannigan and P.S Benson, “Natural Immobilisation of Microorganisms for Continuous Ethanol Production” Enzyme Microb. Technol. Vol. 40, Issue 1, pp. 127‐131,
2006.
[4] A.A Okon and U.T Nwabueze, “Simultaneous effect of divalent cation in hydrolyzed cassava starch medium used by immobilized yeast for ethanol production” African journal of food science, Vol.3, No.8, pp 217‐222 , 2008.
[5] L. Ronghou, L. Jinxia, S.R Fei, “Bioethanol from stalk juice of sweet sorghum by immobilized yeast fermentation” Journal of Renewable Energy,Vol..33, pp 1130‐1135, 2007.
[6] D. Panos D, T.N Elias, L. Maria, “Continuous production of wine in a tower fermentor using entrapped yeast cells in double layer Alginate‐ chisotan beads” Journal of science and technology pp 51‐60 2010.
[7] P. Tataridis, P.V Ntagas, E.T Nerantzis, “Production of sparkling wine with immobilized yeast fermentation” Journal of science of Technology pp1‐18, 2004.
[8] A.T Aikaterini, E. Kalogeris, E. Apostolos, A.T Ali, G. Dimitrios and S. Haralambos, “Effective Immobilization of Candida Antarctica lipase B in organic‐modified clays: Application for the epoxidation of Terpenes” Material Science and Engineering Journal, Vol.165, Issue 3, pp.173‐177, 2009.
[9] V.Ivanova,P.Petrova,J.Hristov,“Application in
the Ethanol Fermentation of Immobilized Yeast Cells in Matrix of Alginate/Magnetic Nanoparticles, on Chitosan‐Magnetite Microparticles and Cellulose‐coated Magnetic
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April‐2013 484
ISSN 2229‐5518
Nanoparticles” International Review of
Chemical Engineering, Vol.3, No.2, pp289‐299,
2011.
[10] Kadambini, G. “Process Optimization for the production of Ethanol via Fermentation.” Department of Biotechnology and Environmental Sciences Tharpar Institute of Engineering and Technology, pp27‐36, 2006.
[11] C. Belkis and F.V Sukan, “Comparison of
Different Production Process for Bioethanol.” Turk. J Chem. Vol. 22, pp.311-32, 1996.
[12] Gough, S. and McHale, A. P. Continuous ethanol production from molasses at 45oC using alginate immobilized Kluyveromyces
marxianus IMB3 in a continuous‐flow
bioreactor. Bioprocess Eng. 19: 33‐36, 1998.
[13] H.Y Wang and D. Hetter, “Cell Immobilization
in K‐Carrageenan with Tricalcium Phosphate” Department of chemical engineering university of Michigan Ann Arbor Michigan,
1982.
IJSER © 2012 http://www.ijser.org





