International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1041
ISSN 2229-5518
Enzymatic mechanism during
phytoextraction of heavy metals from fly ash amended soil
Krishna Rawat, Bhawana Pathak, M.H.Fulekar School of environment and sustainable development Central University of Gujarat
Gandhinagar, Gujarat.
Abstract: A pot culture experiment was carried out to study the phytoextraction potential of selected plant species, for remediation of fly ash contaminated soil. Plant species namely verbena speciosa, tagetes erecta, Cassia tora have been selected for the study. Fly ash samples were collected from coal based thermal power plants at gandhinagar from electrostatic precipitators. Soil samples were collected from Sabarmati river bed. Various physico-chemical parameters of fly ash and soil were conducted. Biomass and biochemical parameters of plants were studied for screening of plants for phytoextraction ability. Study of Photosynthetic pigment, protein content, antioxidative enzymes such as superoxide dismutase (SOD), Ascorbate peroxidase (APX), Catalase (CAT), Guaiacol peroxidase (GPX) was carried out in potential plant. Fly ash heavy metals may lead to oxidative stress which is a condition in which ROS or free radicals are generated extra- or intra-cellularly, which can exert their toxic effects to the cells. However, in cells antioxidant defense mechanisms is present to detoxify the harmful effects of ROS. The plant species were found in order verbena speciosa< tagetes erecta<Cassia tora for phytoremediation of fly ash heavy metals. Tanslocation factor and bioaccumulation factor was also determined. Results showed that cassia tora survived well in fly ash amendments and can be used well for phytoremediation and revegetation of fly ash effected sites.
Keywords: Antioxidative Enzymes, Phytoextraction, Fly Ash, Revegetation.
Introduction
—————————— ——————————
leachability etc. FAUP, TIFAC estimates
that annual ash generation is expected to
In energy production coal serves to be a
major fuel generates by-product named fly ash which is of great concern due to its fine particle size. This fly ash contains various toxic metals more in concentration than in respective coal, thus acts as environment pollutant. Various estimates indicate that in upcoming year’s fly ash production will be increasing manyfolds. Due to this its management is of prime concerns in the country like India where availability of land is less for ash disposal. Also there is risk of
other associated problems like metal
reach about 225 million tonne by 2017 (Vimal Kumar, 2005). According to the international energy outlook 2013(Figure1) though renewable sources and natural gas are the fastest growing sources in electricity generation, still coal will be major fuel for electricity generation.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1042
ISSN 2229-5518

Figure1: Share of resources for electricity generation in coming years
Chemical nature of fly ash comprises of mainly SiO2 , Al2 O3 and oxides of iron and other toxic metals. FA has a low bulk density, high surface area and light texture (Asokan et al., 2005; Jala and Goyal, 2006). Generally FA contains potentially toxic elements such as As, Cu, Zn, Cd, Pb, Ni, Cr, Se, etc. (Rautaray et al., 2003; Lee et al.,
2006; Tiwari et al., 2008; Adriano et al.,
2002). Depending upon the concentrations heavy metals may either inhibit or stimulate antioxidative enzymes activity. Various free radicals are generated due to heavy metals toxicity these may lead to oxidative stress. To overcome environmental issues arising out of fly ash phytoremediation technology is used to monitor dispersal of fly ash into environment and to develop bioaesthetic
environment for local inhabitants. In this
suitable plant species is used to revegetate fly ash dumpsites to accumulate fly ash heavy metals. Plants develops an antioxidative stress which comprises of various enzymes such as superoxide dismutase (SOD), Catalase (CAT), Guaiacol peroxidase (GPX), Ascorbate peroxidase (APX) etc. to overcome this stress.
Present study aim at finding suitable plant species which can easily colonize in fly ash, so that fly ash pollutant can be checked. Phytoextraction appears an encouraging science but it is necessary to transform this science into working system. For determination of real potential of this technology its land implication is important. Though a lot of work is done on phytoremediation yet in countries like India this technology is still in its starting stage, and search for new plant species still remains continue.
Material method
Sample collection
Samples of fly ash were collected from Thermal power station Gandhinagar Gujarat located at 23◦14ꞌ 57″ N latitude and 72◦ 40ꞌ
15″E longitude. Samples of soil were collected from a depth of about 30 cm along the banks of Sabarmati River, Gandhinagar. Stones and plant tissues were carefully
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1043
ISSN 2229-5518
removed from the soil prior to drying in the laboratory. The soil was screened through 2 mm stainless steel sieve and stored in a plastic bag at room temperature until use. Seeds of three ornamental plants namely Cassia tora, Verbena speciosa, Tagetes erecta were chosen for present study. Seeds of these plants were collected form nursery. Experimental set up
Pot culture experiments were conducted
using soil and soil amended with different concentration of fly ash. Fly ash was uniformly mixed with air dried soil sieved to
<2 mm and placed in pots. Various amendments made were 0%, 25%, 50%,
75%, 100% respectively. Twenty seed were sown in soil to germinate. Experiment was conducted in triplicates under natural light and ambient temperature. No artificial fertilizers or soil amendments were added to soil during course of the experiment. Plants were water regularly with water obtained from Millipore water purification system. Physicochemical analysis
The physicochemical parameters were
measured by standard methods. The physico-chemical parameters analyzed were: pH, P, Total nitrogen, Ammonia, Nitrite, Nitrate, Alkalinity, Moisture content, Bulk density, Porosity, Sulphate, Conductivity,
water holding capacity, Cation exchange
capacity was determined after extraction with ammonium acetate at pH 7.0 and organic carbon was determined using the Walkley Black method. Concentrations of Pb and Cd were determined by atomic absorption spectrophotometer (APHA,
1998).
Photosynthetic parameters
Arnon (1949) formulated formula was used for calculating chlorophyll content in plants under study and Kirk and Allen (1965) for carotenoid contents.
Estimation of protein
The protein content was estimated by following the method of Lowry et al., (1951).
Enzymes assay
Superoxide Dismutase was estimated by method of Poonam et al., 1984. Catalase was estimated in the plant parts following the method of Aebi (1984). The ascorbate peroxidase activity was determined in plant by the method of Nakano and Asada (1981). Guaiacol peroxidase activity was determined following the formation of tetraguaiacol as described by Singh et al., (2006).
Statistical Analysis
Physiological, biochemical (photosynthetic pigments, protein content) parameters of plant, heavy metal concentrations of soil
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1044
ISSN 2229-5518
under different treatments were analyzed using one-way analysis of variance (ANOVA) to compare the means of different treatments.
Results and discussion
Fly ash, a by-product of coal combustion, is generated in large quantities in coal- based thermal power plants. Disposal of huge amount of fly ash is of prime concern because of its toxic nature. Phytoremediation is projected as a cost- efficient and ecologically benign process that uses plants to remove heavy metals from the environment by accumulation or transformation of these metals in vegetative biomass. Soil tests tell us condition of soil. Physico-chemical characteristics of soil is essential for the successful growth of plant for remediation of polluted soil.
Physico-chemical properties of the soil fly ash, and its various amendments
Physico-chemical parameter of fly ash
amended in soil (Table 1) showed the increasing trend with the increasing concentration of fly ash in soil parameters such as pH, EC, TDS, Alkalinity,
water holding capacity, porosity of different amendments were increasing as compared to pure soil. While some parameters value decreased includes organic carbon, moisture
content, Cation exchange capacity, bulk
density. Nutrient content also found decreases with increasing concentration of fly ash in soil, nitrogen and phosphorous low concentration coupled with lack of organic content in fly ash mark it as a nutritive deficient substrate. Gupta et al., (2007) reported that with an increase in fly ash amendment ratio pH increased significantly this might be due to the precipitation of soluble cations in the fly ash amended soil. Fly ash amendment to soil significantly increased the electrical conductivity of the soil mixture by increasing the levels of soluble major and minor inorganic constituents (Eary et al.,
1990). Fly ash itself is not effective in retaining water but it significantly increases water holding capacity of the soil mixture (Chang et al., 1977). Fly ash+soil medium has been reported for better soil porosity and thus increased water holding capacity (Gupta et al., 2002) and decreased bulk density (Page et al., 1979). While value for some parameters has decreased like organic carbon, moisture content, Cation exchange capacity, bulk density. The reduced CEC value indicates that fly ash treatment caused change in the mineralogy of treated soil. The result of the present study was similar to earlier finding showing decrease in CEC with increase of fly ash incorporation
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1045
ISSN 2229-5518
(Amrhein et al., 1996; Nalbantoglu, 2004; Sinha and Gupta, 2005; Gupta et al., 2007). Decrease in ammonium nitrogen may be due to the change in pH. Nutrient content also
decreases with increasing concentration of
fly ash, low levels of nitrogen and phosphorous coupled with lack of organic content in fly ash mark it as a nutritive deficient substrate.
Table 1 Physico-chemical properties of soil, fly ash, and its various amendments
Concentration | 0% | 25% | 50% | 75% | 100% |
Parameters | Brown | Light brown | Greyish brown | Light gray | Gray |
Colour | Brown | Light brown | Greyish brown | Light gray | Gray |
Ph | 7.08±.01 | 7.8±.01 | 7.84±.02 | 7.96±.01 | 9.03±.03 |
EC | 186.05±.01 | 192.675±.01 | 234.5±.12 | 354±.01 | 429.25±.02 |
TDS | 80.4±.01 | 95.675±.01 | 110.3±.02 | 124.25±.01 | 178±.01 |
Organic carbon | 0.84±1.4 | 0.7±.01 | 0.63±.01 | 0.59±.02 | 0.54±.01 |
Alkalinity | 9.5±.05 | 16±.01 | 36±.01 | 50±.01 | 61.5±.04 |
Moisture content | 1.1238±3.5 | 0.4396±.01 | 0.3568±.02 | 0.2986±.01 | 0.175±.01 |
WHC | 35.87±.19 | 36.027±.13 | 36.427±.11 | 37.79±.12 | 39.27±.15 |
CEC | 3.304±.20 | 2.38±.53 | 2.254±.07 | 0.504±.01 | 0.266±.01 |
BD | 1.2938±.01 | 1.23581±.01 | 1.1428±.04 | 0.89952±.03 | 0.7404±.02 |
Porosity | 46.5±.01 | 48.71±.02 | 49.83±.01 | 53.98±.02 | 58.13±.02 |
Sulphate | 22.955±.13 | 16.42±.16 | 15.92±.12 | 12.37±.13 | 6.56±.16 |
Ammonia | 8.9±.23 | 0.504±.22 | 0.347±.28 | 0.261±.21 | 0.219±.22 |
Nitrate | 19.9±.95 | 19.12±.93 | 17.7±.93 | 14.45±.95 | 12.00±.90 |
Nitrite | 3.26±.007 | 1.26±.05 | 1.08±.07 | 0.21±.05 | 0.148±.002 |
Phosphate | 14.06±.005 | 11.05±.031 | 9.12±.059 | 8.54±.066 | 1.14±.055 |
All values are mean of triplicates ±S.D. (EC- electrical conductivity, TDS- total dissolved solids, WHC- water holding capacity, CEC cation exchange capacity, BD- bulk density).
coal. Chemical composition of fly ash
Fly ash composition
Fly ash emissions from a variety of coal combustion show a wide range of composition. Variability is directly related to the source of the coal from which it is obtained, its pretreatment processes and the
operation opted in the plant for burning the
showed the dominance of silica followed by alumina and iron oxides. Decreasing order of chemical compound found in fly ash is as follows: SiO2, > Al2 O3, > FeO3 +Fe3 O4, > MgO, > KO2, > CaO, > SO4, > NaO2, > SO3,
> P2 O5. > TiO2 (Table 2).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1046
ISSN 2229-5518
Table 2 Chemical composition of fly ash
0.6
0.5
0.4
0.3
0.2
0.1
0
0 25 50 75 100
Amendments(%)
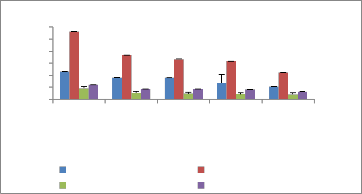
Fresh root weight Fresh shoot weight
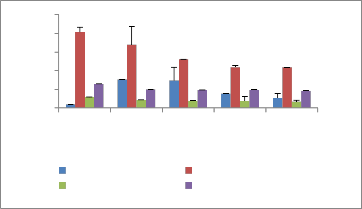
Dry root weight Dry shoot weight
2.5
2
1.5
1
0.5
0
0 25 50 75 100
Amendments(%)
Fresh root weight Fresh shoot weight
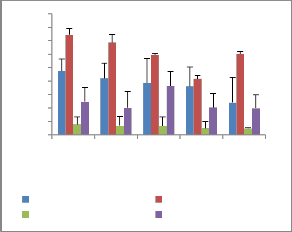
Dry root weight Dry shoot weight
Source: Gandhinagar Thermal Power Plant
Biomass and biochemical parameters
Results demonstrate that with the increasing
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
0 25 50 75 100
Amendments(%)
concentration of fly ash in soil physiological traits like fresh and dry biomass, showed significant retardation (Figure 2). Dose dependent growth responses, both positive and negative in relation to fly ash application have been earlier observed by several workers (Singh and Siddiqui, 2003;
Mittra et al., 2005).
Fresh root weight Fresh shoot weight
Dry root weight Dry shoot weight
Figure 2: Biomass of selected plants after one month in fly ash amended soil
a. Biomass of verbena speciosa b. Biomass of
Tegetes erecta c. Biomass of Cassia tora
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1047
ISSN 2229-5518
The levels of chlorophyll in the plant species also decreased when the concentration of fly ash increases in soil. Results of Chlorophyll a content of Verbena species, revels that with the increased concentration of fly ash initially chlorophyll a concentration increases up to 25 % later on it starts to decreases. While for chlorophyll b and total chlorophyll content value decreased with increased concentration of fly ash. Means were not found significantly different (P value< 0.5080) as determined by one way ANOVA and there was inverse correlation among data. Tagetes, revealed similar trend as verbena. In Cassia tora Chlorophyll b showed increased concentration at 25% then value decreased while chlorophyll a and total chlorophyll showed decreasing trend with increased value (Table3). Decrease in chlorophyll content in fly ash grown plants may be either due to reduction synthesis of chlorophyll or its accelerated degradation. Toxic effect of heavy metals on photosynthesis is also reported by (Morita et al., 2006; Wang et al., 2004). Heavy metals are known to interfere with chlorophyll synthesis either through direct inhibition of an enzymatic step or by inducing deficiency of an essential nutrient (van Assche and Clíjsters 1990). Further d amino-levulinic
acid dehydratase(ALAD) is a metal sensitive
enzyme of chlorophyll biosynthesis which might have been affected by the higher amount of heavy metals in the fly ash thus reducing the chlorophyll content in the plant leaves. Under metal stress reduced ALAD activity (Pb, Cd, Cr, Hg) has been reported earlier in different plant (Vajpayee et al.,2000).
Carotenoid content of all plants was found to be increased with the increasing concentration of fly ash and exposure time. Increased carotenoid content has been considered as defence strategy of the plant to combat metal stress (Kenneth et al.,
2000). Here it may be strategy adopted by plant to counteract the toxic effect of free radicals generated under fly ash stress conditions. Carotenoids (non-enzymatic antioxidant) play a significant role in the protection of chlorophyll pigment under stress conditions by quenching the photodynamic reactions, replacing peroxidation and collapsing of membrane in chloroplasts (Kenneth et al., 2000). According to Tomar et al., (2000), the protein content (Table 3) under heavy metal stress may be affected due to (i) Enhanced protein hydrolysis resulting in decreased concentration of soluble proteins (Melinchuk et al., 1982). (ii) Catalytic
activity of heavy metals (Bhattacharya and
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1048
ISSN 2229-5518
Choudhari, 1997) and (iii) Protein Synthesis becoming reduced under all stress
conditions. All the amendments of fly ash
also showed inhibitory effect on protein content in all selected three plants species.
Table 3 Biochemical parameters of selected plant species
Biochemical parameters | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Caratenoid content | Protein content |
Plants | concentration | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Caratenoid content | Protein content |
Verbena speciosa | 0% | 1.22 | 0.76 | 1.99 | 0.28 | 0.60 |
Verbena speciosa | 25% | 1.24 | 0.55 | 1.79 | 0.56 | 0.41 |
Verbena speciosa | 50% | 1.08 | 0.52 | 1.60 | 0.64 | 0.37 |
Verbena speciosa | 75% | 0.98 | 0.40 | 1.39 | 0.73 | 0.32 |
Verbena speciosa | 100% | 0.70 | 0.24 | 0.94 | 0.92 | 0.26 |
|
Tagetes erecta | 0% | 0.82 | 0.49 | 1.32 | 0.25 | 1.04 |
Tagetes erecta | 25% | 0.92 | 0.41 | 1.33 | 0.40 | 0.31 |
Tagetes erecta | 50% | 0.82 | 0.39 | 1.21 | 0.50 | 0.27 |
Tagetes erecta | 75% | 0.75 | 0.35 | 1.11 | 0.83 | 0.25 |
Tagetes erecta | 100% | 0.61 | 0.31 | 0.93 | 1.51 | 0.24 |
|
Cassia tora | 0% | 1.59 | 0.63 | 2.23 | 0.61 | 1.91 |
Cassia tora | 25% | 1.29 | 0.68 | 1.97 | 0.74 | 1.00 |
Cassia tora | 50% | 1.24 | 0.62 | 1.86 | 0.81 | 0.91 |
Cassia tora | 75% | 1.13 | 0.60 | 1.73 | 1.01 | 0.86 |
Cassia tora | 100% | 0.90 | 0.57 | 1.47 | 1.53 | 0.70 |
Enzymatic mechanism
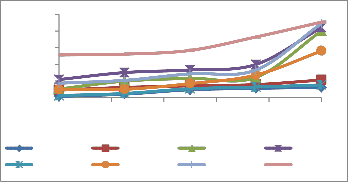
The antioxidative enzymes, guaicol peroxidase, catalase, ascorbate peroxidase, superoxide dismutase, exhibited an increasing trend in response to fly ash heavy metals. Heavy metals caused a significant increase in the activities of enzymes studied. Enzymatic activity in roots was found higher as compared to shoot (Figure 3). Heavy metals are known to generate toxic reactive
oxygen species (ROS) like O2-, OH-, H2 O2 ,
etc. which degrade important cellular
components by inducing oxidative stress (Panda 2003). The level of reactive oxygen species (ROS) in plant tissues is controlled by an antioxidant enzymes (guaicol peroxidase, catalase, ascorbate peroxidase, superoxide dismutase) and non enzymatic low molecular weight antioxidants (glutathione,proline,carotenoid,tocopherols etc.)(Schutzendubel and Polle, 2002). The results of the present study showed all the tested concentrations of fly ash caused
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1049
ISSN 2229-5518
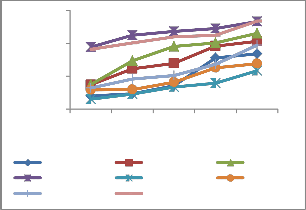
oxidative stress. As fly ash concentrations increased, SOD, CAT and POD, guaicol peroxidase activity enhanced progressively.
3
10
8
6
4
2
0
0 20 40 60
Co ntrati f fly )
80 100
nce
on o
ash(%
2
1
0
0 25 50 75 100
Concentration of fly ash(%)
r 3rd day r 7th day r14th day r 21st day s 3rd day s 7th day s 14th day s 21st day
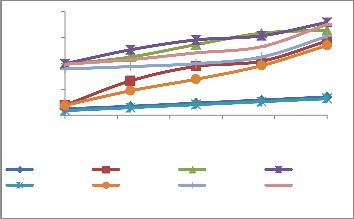
Figure: 3(c) Ascorbate peroxidise activity in plant roots and shoot
2
1.5
1
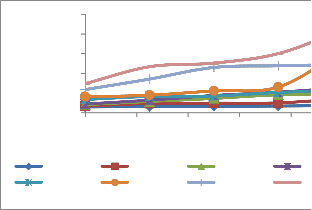
Figure: 3(a) Guaicol peroxidise activity in plant shoot(s) and root(r)
2.5
0.5
0
0 20 40 60 80 100
Concentration in fly ash(%)
2
1.5
1
0.5
0
0 20 40 60 80
Concentration of fly ash(%)
r 3rd day r 7th day r 14th day r 21st day
s 3rd day s 7th day s 14th day s 21st day
Figure: 3(d) SOD activity in plant shoot and root.
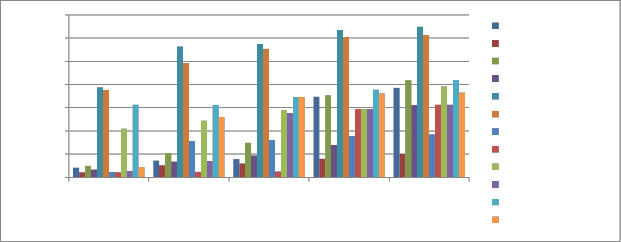
Heavy metal uptake by selected plants
With the increasing concentration of fly ash
Figure: 3(b) Catalase activity in plant root and shoot
heavy metal concentration was found increased in roots and shoots. Concentration of metal was high in roots as compared to shoots (Figure 4). Among selected three plant species i.e Verbena, Tagetes, Cassia. Cassia was found to have maximum heavy metal concentration. Cadmium uptake was
more compared to lead uptake in cassia tora
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1050
ISSN 2229-5518
specie, similar trends were seen in verbena and tagetes roots at 75% and 100% concentration. This can be explained by increased metal availability. While at concentration 0%, 25%, 50% lead uptake was more in tagetes roots. In verbena roots at 0% Cd uptake was more compared to Pb while at 25%, 50% Pb>Cd. In tagetes shoot Pb uptake was higher than Cd accumulation
with the increasing concentration. This can
be explained by more translocation of Pb from roots to shoots of plants. In verbena shoots at 0% concentration Cd and Pb uptake was almost same at 25% 50% concentration Cd uptake was high compared to Pb. But75% and 100% concentration Pb > Cd. Metal accumulation in the plants is depended on its mobility and availability in the soils and plant species growing on these
soils (Sinha and Gupta, 2005).
3.5
3
2.5
2
1.5
1
0.5
0
0 25 50 75 100
Fly ash amendments(%)
Cd in verbena root Cd in verbena shoot Cd in tegetes root Cd in tagetes shoot Cd in cassia root
Cd in cassia shoot Pb in verbena root Pb in verbena shoot Pb in tagetes root
Pb in tagetes shoot
Pb in cassia root
Pb in cassia shoot
Translocation factor
Figure 4 Heavy metal uptake by selected plants
from root to shoot. Result showed a TF<1 suggesting that Pb could not be effectively
The rate of TF value ranged from 0.231 to
0.972 (Table 4) which represents translocation of Cd was effectively made
translocated from the roots to the shoots except in verbena plant in 75% and 100% concentration with values 1.64 and 1.68.

Table: 4 Translocation factor
Concentration | Cadmium | Lead |
Concentration | Verbena | Tagetes | Cassia | Verbena | Tagetes | Cassia |
0% | .522 | .691 | .972 | .967 | .135 | .140 |
25% | .723 | .659 | .875 | .156 | .286 | .829 |
50% | .776 | .635 | .968 | .157 | .954 | .997 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1051
ISSN 2229-5518
75% | .231 | .390 | .953 | 1.64 | .997 | .957 |
100% | .258 | .744 | .945 | 1.68 | .798 | .872 |
Bioaccumulation factor
Results show that bioaccumulation factor of cassia was greater than tagetes and verbena.
In present research BAF of Cd and Pb had a
range between .139- 1.678 and .054-.712
respectively (Table 5). This suggests a good performance of C. tora for phytoremediation of Cd, Pb-contaminated soil.
Table: 5 Bioaccumulation factor
Concentration  | Cadmium | Lead |
Concentration  | Verbena | Tagetes | Cassia | Verbena | Tagetes | Cassia |
0% | .139 | .182 | 1.678 | .054 | .288 | .433 |
25% | .179 | .250 | 1.533 | .183 | .321 | .584 |
50% | .192 | .342 | 1.569 | .191 | .573 | .699 |
75% | .577 | .661 | 1.665 | .457 | .569 | .712 |
100% | .638 | .957 | 1.659 | .430 | .609 | .678 |
Difference in uptake and translocation of heavy metals is important in defining their potential utilization in the different phytoremediation procedures. All selected plant species were tolerant to heavy metal contamination of fly ash. Among them, the selected species cassia had good biomass production and could be considered more efficient for phytoextraction purposes.
Conclusion
Combustion of coal in thermal power plant continues to be the main source of energy production all over the world since past few
decades. Due to which huge amount of fly
ash is generated. This fly ash is harmful as it
contains various toxic heavy metals and some radioactive substances. Thus disposal and utilization of fly ash is of prime concern. Complete utilization of fly ash could solve the problem associated with overflow of fly ash and its exposure to surrounding environment and its impact on human health and environment and also minimize the burden on land requirement for disposal.
The outcomes of the study will be helpful to control the human health hazards and effective conversion of wasteland, which is of paramount importance to an overpopulated country like India as well as open an avenue in solving coal ash disposal
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1052
ISSN 2229-5518
problem to avoid the environment degradation.
The present study was undertaken to find out the potential plant for remediation of soil contaminated with fly ash and to study various enzymatic activity in potential plant. It was observed that Cassia tora (Caesalpinioideae) has potential to remove contaminant in fly ash amended soil polluted by various heavy metals resent in fly ash. Several important aspects of metal tolerance in selected plant i.e Cassia tora has been identified.
Acknowledgment
I would like to express my deepest gratitude to my supervisor Prof. M.H.Fulekar. Special thanks to Dr. Bhawana Pathak (Asstt. Prof.). I owe a deep sense of gratitude to Prof. R.K. Kale, Vice- Chancellor, Central University of Gujarat for providing all the support and environment for research work. I thanks my parents Mr. D.R.Rawat and Mrs. Geetanjali Rawat and other family members and friends support and encouragement. Lastly I would like to acknowledge RGNF fellowship for providing me financial support for my research work.
References
1. Aebi HE. Catalase. In: Bergmeyer J, Grassl M, editors 1983. Methods of
Enzymatic Analysis. III, Enzymes: Oxidoreductases, Transferases. Wienheim, Germany: Verlag Chemie;. pp. 273–286.
2. Ahmaruzzaman, M. 2010. “A review on the utilization of fly ash”, Progress in Energy and Combustion Science, Vol. 36(3), pp 327-363, June.
3. Amrhein, C., Haghnia, G. H., Kim, T. S.,Mosher, P. A., Gagajena, R. C., Amanios, T.,and Torre, T. D. L.,
1996. Synthesis andProperties of Zeolites from Coal Fly Ash, Environ. Sci. Technol., 30(3), 735-742.
4. APHA American Public Health Association 1998. Standard methods for examination of water and waste water. 20th Edition, New York,USA.
5. Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol., 24, 1-15.
6. Asokan, P.; Saxena Mohini; Asolekar Shyam R. 2005. Coal combustion residues environmental implications and recycling potentials Resources Conservation and Recycling - RESOUR CONSERV RECYCL , vol. 43, no. 3, pp. 239-
262.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1053
ISSN 2229-5518
7. Baba 2002. Assessment of radioactive contaminants in by products from Yatagan coalfired power plant. Environmental Geol 41:
916-921.
8. Bhattacharya, M., Choudhuri, M. A.
1997. Effect of lead and cadmium on the biochemical changes in the leaves of terrestrial (Vigna) and aquatic (Hydrilla) plants under solution culture Indian J. Plant Physiol. 32. 99-103.
9. Burd, G.I., D.G. Dixon and B.R.
Glick, 2000. Plant growth promoting bacteria that decreases heavy metal toxicity in plants. Can. J. Microbiol.,
46: 237-245.
10. Chang, A.C., Lund, L.J., Page, A.L., Warneke, J.E., 1977. Physical properties of fly ash amended soils.J.Environ.Qual.6 (3), 267-270.
11. Eary, L. E., Rai, D., Mattigod, S. V., and Ainsworth, C. C. 1990. Geochemical factors controlling the mobilization of inorganic constituents from fossil fuel combustion residues. Ii. Review of the minor elements. J. Environ. Qual., 19,202-214.
12. Gupta, A.K., Dwivedi, S., Sinha, S.,
Tripathi, R.D., Rai, U.N. & Singh
S.N. 2007. Metal Accumulation and growth performance of Phaseolus vulgaris grown in fly ash amended soil. Bioresour. Technol., 98:3404-
3407.
13. Gupta, D. K., Rai, U. N., Tripathi, R.
D., and Inouhe, M. 2002. Impacts of flyash on soil and plant responses. J.Plant Res., 115, 401-409.
14. Jala Sudha and Goyal Dinesh 2006.
Fly ash as a soil ameliorant for improving crop reduction--a review. Bioresource Technology, 97(9),
1136-47.
15. Kenneth, E., K.E. Pallet and J.
Young 2000. Carotenoids. Antioxidants in higher plants. Kath, G. Alscher (Eds.) John L. Hess. CRC Press, Boca Raton, Florida USA, pp.
60-81.
16. Khan, R., Mujeebur & Khan, W.M.
1996. The effect of fly ash on plant growth and yield of Tomato. Environ. Poll., 92: 105-111.
17. Lowry, O.H., Rosenbrough, R.J., Farr, A.L. and Randall, R.J. 1951. Protein measurement with folin phenol reagent. J. Biol. Chem., 193,
265–275.
18. Melinchuk, Y Lishko AK and
Kalinin FI 1982. Cadmium effect on
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1054
ISSN 2229-5518
free amino acid content in germs of pea seeds at early germination stages. Fiziologia Biokhimiya Kuliurnyh Rastnii 14 385-395.
19. Mitrra BN, Karmakar S, Swain DK, Ghosh BC. 2005 Fly ash—a potential source of the soil amendment and a component of integrated plant nutrient supply system. Fuel 84:1447–51.
20. Morita, A., H. Yokota, M.R. Ishka and F. Ghanati 2006. Changes in peroxidase activity and lignin content of cultured tea cells in response to excessmanganese. Soil Sci. Plant Nutr., 52, 26-31.
21. Nakano Y, Asada K. 2005.
Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol.22:860–867.
22. Nalbantoglu, Z., 2004. Effectiveness of class C fly ash as an expansive soil stabilize. Construction and Building Materials 18(6), 377381.
23. Page, A. L., A. A. Elseewi and I. R.
Straughan. 1979. Physical and Chemical Properties of Fly Ash from Coal-Fired Power Plants with Reference to Environmental Impacts.
Residue Reviews 71:83-120.
24. Panda, S.K. 2003. Heavy metal phytotoxicity induces oxidative stress in a moss, Taxithellium sp., Curr. Sci., 84 (5), 631-633.
25. Poonam K, Ballabh D,Vishwanathan PN. 1984. A modified spectrometric assay of superoxide dismutase. Ind J Biochem Biophysics 21: 130-132.
26. Rajkumar, M., R. Nagendran, K.J.
Lee, W.H. Lee and S.Z. Kim, 2006. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere, 62:
741-748.
27. Raskin, I., Smith, R. D. & Salt, D. E.
1997. Phytoremediation of Metals: Using Plants to Remove Pollutants from the Environment; Current Opin. Biotechnology, Vol. 8, 221-226.
28. Salt, D.E., Smith, R.D. and Raskin, I.
1998. Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology,49: 643-
668.
29. Singh, N., Ma, L. Q., Srivastava, M.,
& Rathinasabapathi, B. 2006. Metabolic adaptations to arsenic- induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Science, 170, 274–282.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1055
ISSN 2229-5518
30. Sinha, S., Gupta, A.K., 2005.
Translocation of metals from fly ash amended soil in the plant of Sesbania cannabina L. Ritz: effect on antioxidants. Chemosphere 61,
1204–1214.
31. Singh, L.P. and Z.A. Siddiqui,
2003 Effects of flyash and Helminthosporium oryzae on growth and yield of three cultivars of rice. Bioresour. Technol., 86: 73-78.
32. Schützendübel A, Polle A 2002.
Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53: 1351–1365.
33. Tomar, M.; Kaur, I.; Neelu; Bhatnagar A.K. 2000. Effect of enhanced lead in soil on growth and development of Vigna radiata (L.) Wilezek. Indian J. Plant Physio. 5(1):
13-18.
34. Van Assche F., Clijsters, H., 1990.
Effect of metals on enzyme activity in plants. Plant Cell Environ. 13,
195–206.
35. Vajpayee, P., Tripathi, R.D., Rai, U.N., Singh, S.N., 2000. Chromium
Nymphaeaalba. Chemosphere 41,
1075–1078.
36. Wang, S.H., Z.M. Yang, H. Yang, B.
Lu, S.Q.Li and Y.P.Lu 2004. Copper induced stress and antioxidative responses in root of Brassica juncea L.Bot. Bull. Acad. Sin., 45,203-212.
accumulation | reduces | chlorophyll |
biosynthesis, | nitrate | reductase |
activity and | protein | content of |
IJSER © 2015 http://www.ijser.org








