The research paper published by IJSER journal is about Electrophoretic Deposition of Silica on Stainless Steel 1
ISSN 2229-5518
Electrophoretic Deposition of Silica on Stainless
Steel
Ryan D. Corpuz, Lyn Marie Z. De Juan, Herman D. Mendoza, Meliton U. Ordillas
Abstract—Fabrication of stainless steel-silica composite was successfully done using Direct Current Electrophoretic Deposition. Deposition of silica particles on stainless steel substrate was conducted in an aqueous suspension with 0.001M Sodium Nitrate as background electrolyte, 30V constant voltage and a deposition time of 5 and 10 minutes. Characterization of the samples s howed randomly distributed silica particles for 5 minutes deposition time and aggregated silica particles for 10 minutes deposition time.
Index Terms— particle size analysis, scanning electron microscopy, EPD
—————————— ——————————
LECTROPHORETIC DEPOSITION was discovered by a Russian Scientist named Rues in early 19th century in his experiment with electric field to induce motion of clay particles suspended in water [1]. First commercialization of this method is patented in the USA in early 1930’s with the deposition of thoria on a platinum cathode for electron tube applications and only in 1980’s did the method received unique recognition for fabrication of advanced ceramic mate- rials [2]. Important applications of these electrophoretically deposited advanced ceramic materials could be found in fuel cells, optics, bioceramics, photonics, and electronics. Aside from ceramic materials [3], this method had already been adopted in almost all materials like metals [4], polymers [5]
and composites [6].
Advantages of EPD method lie in its simplicity and inexpen- siveness. EPD set up could easily be fabricated using low costs materials. The simplest design consists only of two parallel conducting plates as electrodes, container of suspension, and dc battery, as voltage source to facilitate migration of particles. Aside from simplicity and inexpensiveness, EPD is a fast me- thod which made it attractive in industries for it is suitable for mass production.
The objective of this study is to fabricate a composite material consists of metal and ceramic oxide. In particular, the re- searcher investigated the possibility of using electrophoretic deposition method in fabricating a composite material made up of stainless steel and silica. It is known that both materials have important biomedical applications [7-9] and its compo- site is an important high temperature material [10-11]. A fast
————————————————
![]() Ryan D. Corpuz is currently pursuing Masters Degree program in Mate- rials Science and Engineering in Universityof the Philippines- Diliman,Philippines, and a faculty member of Mindanao State University – Illigan Institute of Technology, Ceramics,Chemical and Metallurgical En- gineering Department, PH-+639277758935.
Ryan D. Corpuz is currently pursuing Masters Degree program in Mate- rials Science and Engineering in Universityof the Philippines- Diliman,Philippines, and a faculty member of Mindanao State University – Illigan Institute of Technology, Ceramics,Chemical and Metallurgical En- gineering Department, PH-+639277758935.
E-mail: corpuzryan22@yahoo.com.ph
![]() Lyn Marie Z. De Juan is currently pursuing Masters Degree program in
Lyn Marie Z. De Juan is currently pursuing Masters Degree program in
![]() Herman D. Mendoza is a Doctor of Engineering and a professor in the
Herman D. Mendoza is a Doctor of Engineering and a professor in the
University of the Philippines-Diliman, Department of Minning, Metallur-
![]()
gical, and Material Engineering, PH-+639209031273. E-mail: judge.mendoza@yahoo.com
method suitable for mass production of stainless steel-silica composite is
Industrial grade 98% purity silica powder from Dalisay Phil- ippines Corporation was prepared by sieving the particles using 270, 325 and 400 meshes in a mechanical shaker prior to suspension preparation. Five grams (5g) of sample from each mesh were then analyzed using Coulter Counter to evaluate its particle sizes and distribution.
Suspension for Electrophoretic Deposition was prepared by weighing 50 mg of the analyzed silica powder and then pour- ing it in a 1L 0.001 M Sodium Nitrate Solution. The resulting suspension was then ultrasonicated for 6 minutes prior to Electrophoretic Deposition to prevent aggregation and settling of particles.
Electrophoretic deposition was done by setting up rectangular stainless steel electrodes with 10mm x 10mm dimensions, 10 mm apart in a fabricated electrophoretic deposition cell. The ultrasonicated silica suspension was then poured in the EPD cell. After which, a constant voltage of 30V was then applied for 5 minutes and 10 minutes. The sample was then air dried before characterization.
Scanning Electron Microscope was used to view and image the stainless steel substrate and the electrophoretically depo- sited silica. Micrographs for each sample were then taken at
2,000 X and 10,000 X magnifications to view the overall surface
of the sample and its details.
Meliton U. Ordillas is a Professor Emiritus of the University of the Philip- pines-Diliman, Philippines, E-mail: muordillas@yahoo.com
IJSER © 2012
The research paper published by IJSER journal is about Electrophoretic Deposition of Silica on Stainless Steel 2
ISSN 2229-5518

This study focused on the possibility of producing stainless steel-silica composite using electrophoretic deposition me- thod. Deposition of silica particles on stainless steel substrate was done under constant voltage of 30V for 5 and 10 minutes in an aqueous suspension using 0.001M sodium nitrate as background electrolyte. After deposition, samples were then observed and imaged using Scanning Electron Microscope (SEM) to characterize the morphology of the deposited silica particles on stainless steel substrate.
particles on stainless steel after deposition for 5 minutes under 30
V electric potential. It can be observed that these particles are
randomly distributed throughout the surface and it vary in amount and sizes. Of these particle sizes, only a minute amount is coarse and the rest are fine particles.
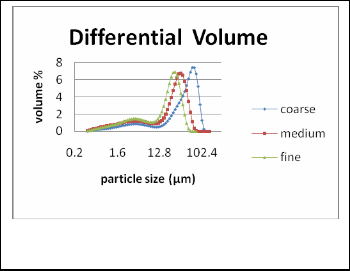
Fig. 3. Electrophoretically deposited silica on stainless steel substrate show- ing aggregated silica particles taken at 2,000 X and 10,000 X magnifications respectively.
Fig. 1. Particle size distribution of silica particles after sieving using 270,
325 and 400 mesh
Industrial grade 98% purity silica powder from Dalisay Phil- ippines Corporation was prepared by sieving the particles using 270, 325 and 400 meshes in a mechanical shaker prior to suspension preparation. Five grams (5g) of sample from each mesh were then analyzed using Coulter Counter to evaluate its particle sizes and distribution.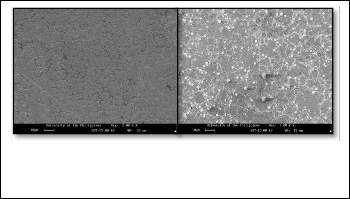
Fig. 2. SEM micrograph showing the surface of stainless steel and the over- all feature of the randomly distributed silica particles on stainless steel sub- strate taken at 2000 X magnification
The SEM micrograph on the left hand side is the surface of stain- less steel substrate before deposition of silica particles. SEM mi- crograph on the right hand side on the other hand shows the silica
The SEM micrograph on the left hand side shows silica par- ticles after 10 minutes deposition under 30V constant voltage. On this micrograph, it can be observed that a larger portion of the deposited particles are fine and only a little amount of large particles are present. Unlike the micrograph for 5 mi- nutes deposition time, these fine particles are aggregated and not randomly distributed throughout the surface of the stain- less steel. A closer view of the micrograph shows these aggre- gations of fine particles (micrograph on the right). Further- more, it is also noticeable that for both figures presented, the deposited silica particles are somewhat equiaxed and could therefore be estimated as spherical particles.
Fabrication of stainless steel-silica composite in an aqueous suspen- sion was successfully done using a direct current electrophoretic deposition with a constant voltage of 30V for 5 and 10 minutes. SEM micrographs of the samples showed randomly distributed silica particles for the former and aggregated silica particles for the latter.
[1] Sarkar, P. and Nicholson, P.S., Electrophoretic deposition (EPD): mechanism, kinetics and application to ceramics. J. Am. Ceram. Soc., 1996, 79; 1987-2002.
[2] Besra, L. and Liu M., A review on fundamentals and applications of electro- phoretic deposition (EPD), Progress in Material Science, 2007, 52; 1-61.
[3] Kaya, C., Kaya, F., Su B., Thomas, B., and Boccaccini A. R., Structural and functional thick ceramic coatings by electrophoretic deposition, Surface and Coatings Technology, 2005, 191; 303 – 310.
[4] Zhang, G., Chen, X., Zhao, J., Chai, Y., Zhuang, and Luyan, W., Electropho- retic deposition of silver nanoparticles in lamellar lyotropic liquid crystal, Mate- rials Letters 2006, 60; 2889–2892.
[5] Tada, K. and Onoda, M., Preparation of flat and dense conjugated polymer films from dilute solutions by means of electrophoretic deposition, Thin Solid Films, 2009, 518; 711–713.
[6] Grandfield, K. and Zhitomirsky, I., Electrophoretic deposition of composite hydroxyapatite–silica–chitosan coatings, Materials Characterization, 2008, 59;
61-67.
IJSER © 2012
The research paper published by IJSER journal is about Electrophoretic Deposition of Silica on Stainless Steel 3
ISSN 2229-5518
[7] Dewidar, M., Khalil, A., and Lim, K., Processing and mechanical properties of porous 316L stainless steel for biomedical applications. Trans. Nonferrous Met. Soc. China, 2007, 17; 468-473.
[8] Li, H., Fu, Y., Zhang, L., Xiuming Liu, X.,Ying Qu, Y., Xu, S. and, Lü, C., In situ route to novel fluorescent mesoporous silica nanoparticles with 8 - hydroxyquinolinate zinc complexes and their biomedical applications, Micropor- ous and Mesoporous Materials, 2012, 151; 293–302.
[9] Gurappa, I., Development of appropriate thickness ceramic coatings on 316 L
stainless steel for biomedical applications, Surface and Coatings Technology,
2002, 161; 70–78.
[10] Takemori, M., Crack formation, exfoliation, and ridge formation in 500 8C annealed sol–gel silica coatings on stainless steel SUS304: Part I. Microscopic observations and elemental analyses, Ceramics International, 2009, 35; 1731–
1746.
[11] Lee, D. W., Lee, Y. G., Sea, B., Ihmb, S. K., and Lee, K. H., Improvement in thermal stability of stainless steel supported silica membranes by th e soaking– rolling method, Journal of Membrane Science, 2004, 236; 53–63.
IJSER © 2012