International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2035
ISSN 2229-5518
Effects of spring and autumn seasons on the variability among sunflower (Helianthus
annuus L.) accessions for pollen viability, germination and morphology
Humera Razzaq1, Shamsa Kanwal1, Muhammad Hammad Nadeem Tahir1 and Bushra Sadia2
1Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad, Pakistan
2Centre of Agricultural Biochemistry and Biotechnology, University of Agriculture, Faisalabad, Pakistan
ABSTRACT: In general exclamation pollen is not competent to maintain the viability and germination for longer time. High temperature has great adverse effects on pollen viability and ultimately it lessens its fertilization ability. In Pakistan there are two sunflower sowing seasons i.e. spring and autumn, having different climatic conditions. The research was conducted in the Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad. Ten sunflower accessions developed and maintained by the Oilseed Research Group, Department of Plant Breeding and Genetics, were evaluated for pollen viability, germination and morphology in spring and autumn seasons. Split plot design with three replications was used for this study. All the accessions, seasons and their interaction showed non-significant difference for pollen viability and highly significant difference for pollen germination. The accession G-33 and G-7 had better performance for both pollen viability and germination under spring and autumn seasons respectively. Autumn season showed higher percentage for pollen viability and germination than spring season. A high percentage of pollen viability and germination helps in seed setting and this may be useful in hybridization, artificial breeding and to study the sterility problems. These selected accessions may be used in breeding for the improvement of economically important achene yield trait.
Key Words: Sunflower; Spring and autumn seasons; Pollen viability; Pollen germination; Pollen morphology
1. INTRODUCTION
Sunflower is the second most important crop worldwide for oil production. Presence of vitamin A, B complex,
D, E, K, calcium and phosphorous [1] in its oil makes healthy cooking oil [2]. Its oil is premium due to light colour, good in taste, high smoke point, oxidative stability, good dietary qualities and high level of unsaturated fatty acids. Sunflower crop is not bound to the season, fits well in the cropping pattern of Pakistan and is grown in spring and autumn seasons. But its achene yield per hectare (1345 kg/ha) is far less than other countries like Turkey (2036.0 kg/ ha), China (1752.6 kg/ha) and USA (1567.1 kg/ha) [3].
Pollen study is required to determine the genetic behaviour, because pollen is the source of genetic variation. As
it is the main vector which inserts all the genetic material in the next generation [4]. Pollen grains comprise a hard coat that guards the sperm cells during their transfer from the stamens to the pistil of flowering plants. After pollen grain transfer on a compatible pistil, germination and production of pollen tube starts which transfers the sperm.
Climate has great effect on the pollen viability and germination in different crops [5]. Pollen grain is prone to heat and humidity in Triticum aestivum L. [6], Sorghum bicolor L. [7] and Hordeum vulgare L. [8]. Unfortunately very little information is reported about the interaction of climate and pollen production in sunflower [9, 10] and between pollen viability and yield in sunflower [11]. Sunflower pollen viability is highly influenced by the climate variation particularly by temperature [12]. Study of pollen production provides the significant knowledge for breeding [13].
The study of pollen is very useful in the breeding programs for artificial pollination procedures [5], creation of sterile lines, hybridization [14] and in the evolutionary ecology [15]. Viable pollen affects the achene yield because only these can fertilize and germinate. A very low rate of viable pollen that loads on stigma to fertilize the ovules can alter the anatomy and development of achene causing reduction in yield [16, 17, 18, 19, 20, 21,
22].
Pollen viability is an important biological trait and is tested by using different stains or by demonstration of
germination percentage. Tetrazolium based stains are also used for the pollen viability test [23]. Colourless
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2036
ISSN 2229-5518
tetrazolium is reduced to coloured formazan by the oxidative capacity of the pollen grains [24] and viable
pollen can be observed by intensity of colour.
Keeping in view the situation current study was carried out with the objective to determine the genetic
variability among sunflower accessions for pollen morphology, viability and germination percentage under spring and autumn seasons.
2. MATERIAL AND METHODS
2.1 Experimental Condition
The research work was conducted in the Department of Plant Breeding and Genetics, University of Agriculture,
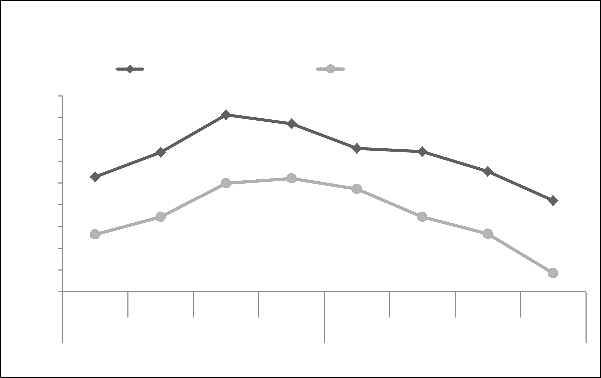
Faisalabad, Pakistan during 2011. Faisalabad is situated in the rolling flat plains of North East Punjab. It is between longitudes 73˚-06́ East, latitude 30˚-26́ North and altitude is 184.4 m, possesses arid climate and loamy soil in field. Average minimum and maximum temperature of spring and autumn crop season in 2011 is presented in Fig. 1 and relative humidity and total rainfall in Fig. 2.
Fig.1 Monthly average maximum and minimum temperatures during spring and autumn crop season of 2011
Maximum temperature(˚c) Minimum temperature(˚c)
45
40
35
30
25
20
15
10
5
0
March April May June September October November December
Spring-2011 Autumn-2011
Months
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2037
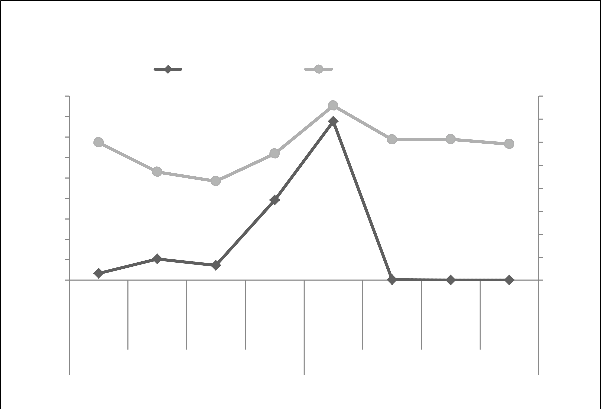
ISSN 2229-5518
Fig. 2 Monthly average relative humidity (%) and total rainfall (mm)
spring and autumn crop season of 2011
180
160
140
120
100
80
60
40
20
0
Total Rainfall(mm) Relative Humidity (%)
80
70
60
50
40
30
20
10
0
Spring-2011 Autumn-2011
Months
2.2 Experimental Material
The experimental material compromised of 10 sunflower accessions viz. HBRS-1, A-48, G-33, G-56, A-23, A-
30, A-45, G-8, G-40 and G-7. The accessions were developed and maintained by the Oilseed Research Group, Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad, Pakistan.
2.3 Experimental Layout
This experiment was laid out under split plot design with three replications during the 2011 spring and autumn
seasons. Crop was grown in spring season on 14 March, 2011 and in autumn season on 8 September, 2011. Row to row and plant to plant distance was maintained 75 cm and 25 cm, respectively.
2.4 Methods
Fresh pollen grains were collected in petri dish from single plant of each accession per replication. Pollen grains
were observed for following parameters.
2.4(a) Pollen viability test
Fresh pollen grains were taken on glass slide in a drop of 0.5% 2, 3, 5 Triphenyl Tetrazolium Chloride (TTC) in the 15% sucrose solution. Sample was covered with cover slip immediately to avoid the oxidation and was
placed in incubator for 2 hours [25]. After this treatment viability was observed by identifying viable pollen
(reddish colour) and non-viable (light brown) under microscope (Olympus 1×50).
2.4(b) Pollen germination
For pollen germination fresh pollen grains were collected and kept in petri dish. A 15% sucrose solution was prepared in a mixture of 50% H3 BO3 (2×10-3M) and 50% Ca(NO3 )2 (6×103M) by volume. Pollen grain sample was taken on glass slide in a drop of sucrose solution and covered to avoid oxidation. This slide was kept in a petri dish lined with wet filter paper and was kept in incubator for 2 hours [25]. The slides were then observed under microscope (Olympus 1×50), pollen grains germinating pollen tubes were counted and percentage was calculated.
2.4(c) Pollen Morphology
The pollen grains of the accessions under study were also observed for pollen shape, colour and appearance by using microscope (Olympus 1×50).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2038
ISSN 2229-5518
2.5 Statistical analysis
The data were analyzed statistically following analysis of variance technique [26] to determine the differences among accessions, seasons and their interaction for pollen viability and germination. Tukey’s test [27] was used
to find the differences among accessions within and between the seasons.
3. Results and Discussion
3.1 Pollen viability
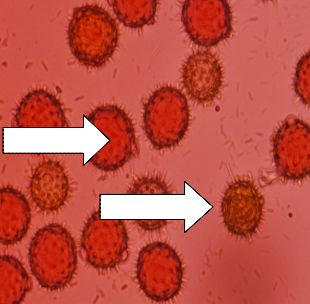
Viable and non-viable pollen grains were differentiated by colour, viable pollen with reddish colour and non-
viable pollen with light brown colour (Fig. 6). Analysis of variance showed that accessions, seasons and their
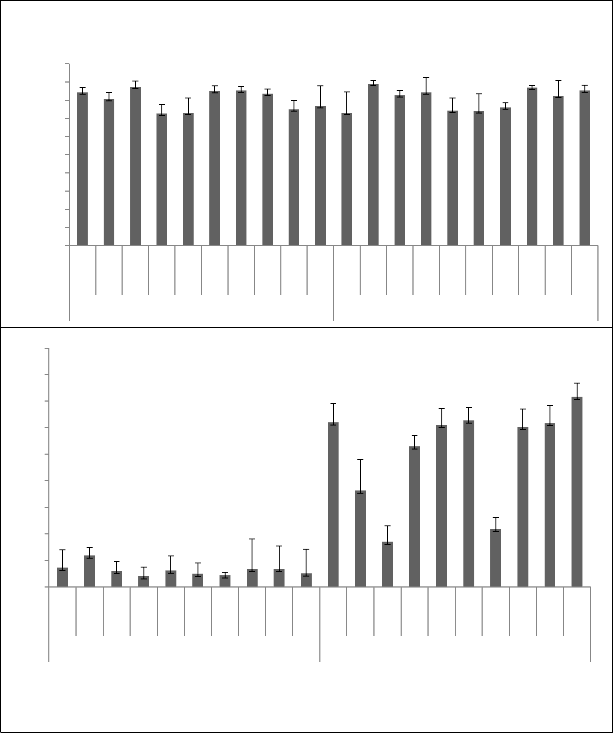
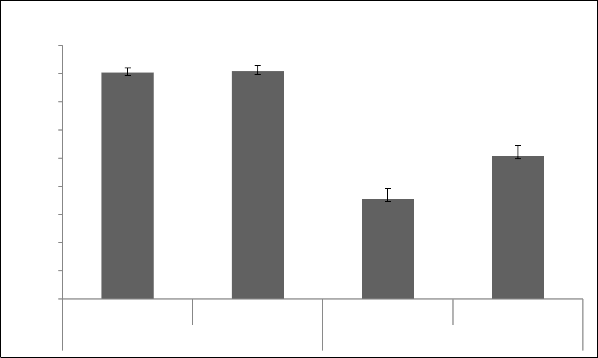
interaction had non-significant differences (Table 1) for this trait. Pollen viability ranged from 72% to 87% in spring season while 73% to 89% in autumn. Pollen viability has been reported from 53.54% to 90.97% in literature [28, 29, 30, 31, 32, 33, 34]. Pollen viability range in our breeding material is comparatively more than the range found in literature. All accessions had more than 75% viable pollen except A-23 and G-56 in spring season and A-30 and HBRS-1 in autumn (Fig. 3). The accession G-33 followed by A-45 and A-30 showed the highest pollen viability percentage under spring season, while accession A-48 followed by G-8 and G-7 in autumn season. Although difference between the growing seasons for viability of pollen grains was non- significant yet, pollen viability percentage was slightly higher in autumn season than spring (Fig. 5).
Table I: Analysis of variance for pollen viability and germination in sunflower accessions
Sources of variation | Pollen viability | Pollen germination |
Replication | 0.22 | 104.4 |
Season | 2.82 | 3450.4** |
Error 1 | 69.6 | 23.12 |
Accessions | 80.7 | 820.9** |
Season x Accessions | 117.3 | 1235.45** |
Error 2 | 116.1 | 135.2 |
** = Highly significant (P<0.01)
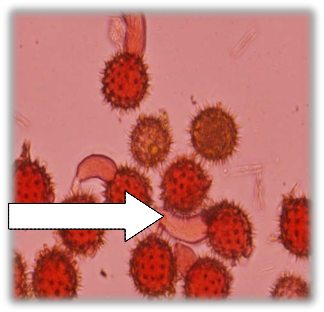
3.2 Pollen germination
Pollen germination was observed by formation of pollen tube (Fig. 7). Only viable pollen grains which are properly transferred on the stigma may germinate pollen tube in the pistil and fertilize the ovules (Connor and
Hall, 1997). Accessions, seasons and their interaction showed significant differences for pollen germination
(Table I). It indicates that seasons had strong effects on the germination of accessions. Pollen germination
ranged from 14% to 57% in spring season whereas 17% to 71% in autumn (Figure 2(b)). Pollen germination range in literature is 40.40% to 74.6% [31, 32, 35]. Pollen germination of breeding material studied in this experiment is comparable with the range found in literature. Accessions showed more than 20% pollen germination except G-56, A-30 and A-45 in spring season. In autumn season all the accessions had more than
35% pollen germination except A-45 and G-33. The accession A-23 followed by G-8 and G-33 showed maximum pollen germination (57%) in spring season and it was significantly different from all accessions except A-48, G-33 and G-8. While G-7 followed by A-30 and HBRS-1 showed highest pollen germination (71.67%) in autumn season and it was non-significantly different from all accessions except A-48, G-33 and A-
45. On the whole pollen germination percentage was higher during autumn growing season compared to that in spring season because in Pakistan the temperature at the time of flowering and pollination during spring is usually higher than that in autumn season which may retard the pollen tube germination [12].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2039
ISSN 2229-5518
100
90
80
70
60
50
40
30
20
10
0
Fig. 3 Pollen viability of sunflower accessions in spring and autumn crop season
Spring Autumn
Fig. 4: Pollen germination of sunflower accessions in spring and
90 autumn
80
70
60
50
40
30
20
10
0
Spring Autumn
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2040
ISSN 2229-5518
Fig.5 Pollen viability and germination in spring and autumn seasons
90
80
70
60
50
40
30
20
10
0
Spring Autumn Spring Autumn
Pollen viability Pollen germination
3.3 Pollen morphology
Samples of pollen grains were examined for the pollen shape, colour and appearance under digital microscope
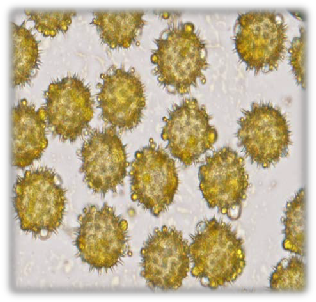
(Olympus 1×50) (Table II). All the accessions had pale yellow colour in autumn season. While in spring season
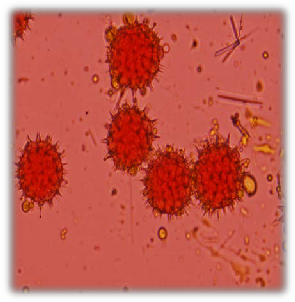
all the accession had pale yellow except G-8, A-30 and G-40 (yellow) (Fig. 8). Golf ball shape and pines pollen appearance were observed for all accessions in both seasons (Fig. 9).
Table II Different pollen characters of studied sunflower accession
Seasons | Accession | Pollen colour | Pollen shape | Pollen appearance |
Spring | HBRS-1 | Pale yellow | Golf ball | Pines |
Spring | A-48 | Pale yellow | Golf ball | Pines |
Spring | G-33 | Pale yellow | Golf ball | Pines |
Spring | G-56 | Pale yellow | Golf ball | Pines |
Spring | A-23 | Pale yellow | Golf ball | Pines |
Spring | A-30 | Yellow | Golf ball | Pines |
Spring | A-75 | Pale yellow | Golf ball | Pines |
Spring | G-8 | Yellow | Golf ball | Pines |
Spring | G-40 | Yellow | Golf ball | Pines |
Spring | G-7 | Pale yellow | Golf ball | Pines |
Autumn | HBRS-1 | Pale yellow | Golf ball | Pines |
Autumn | A-48 | Pale yellow | Golf ball | Pines |
Autumn | G-33 | Pale yellow | Golf ball | Pines |
Autumn | G-56 | Pale yellow | Golf ball | Pines |
Autumn | A-23 | Pale yellow | Golf ball | Pines |
Autumn | A-30 | Pale yellow | Golf ball | Pines |
Autumn | A-75 | Pale yellow | Golf ball | Pines |
Autumn | G-8 | Pale yellow | Golf ball | Pines |
Autumn | G-40 | Pale yellow | Golf ball | Pines |
Autumn | G-7 | Pale yellow | Golf ball | Pines |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2041
ISSN 2229-5518
Viable pollen Non-viable | Germinating pollen |
Fig. 6: Pollen viability reddish colour (viable pollen) and light brown (non-viable pollen) in sunflower accessions | Fig. 7: Pollen germination in sunflower accessions |
| |
Fig. 8: Pollen colour of sunflower accessions | Fig. 9: Pollen shape and appearance of sunflower accessions |
4. Conclusion
Presence of the genetic differences among the accessions for pollen germination in different growing seasons may be exploited in the hybridization program and also in artificial breeding. Performance of sunflower
accessions in both seasons for pollen viability and germination suggests their use in the future to create sterile lines, evolutionary ecology and also in seed setting. The accessions G-33 and G-7 showed better performance
for pollen viability and germination under spring and autumn season respectively. On the whole accessions had better performance in autumn season than spring, it is may be due to high temperature at the flowering and
pollination in spring season in Pakistan which may abort the pollen grain and causing low pollen germination.
Acknowledgment
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2042
ISSN 2229-5518
The authors would like to acknowledge Dr. Faiz Ahmad, Asst. Professor, Centre of Agricultural Biochemistry and
Biotechnology (CABB), University of Agriculture Faisalabad for his unreserved cooperation during this study.
LITERATURE CITED
[1] Reddy, S. R. 2006. Agronomy of Field Crops. 2nd ed. Kalyani Publishers.
[2] Wahid, A., A. Noreen, S. M. A. Basra, S. Gelani and M. Farooq. 2008. Priming-induced metabolic changes in sunflower (Helianthus annuus L.) achenes improve germination and seedling growth. Botanic. Studies
49: 343-350.
[3] FAO. 2013. Food and agriculture organization of the united nations, Rome, Italy.
[4] Dafni, A. and D. Firmage. 2000. Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Systematics and Evolution 222:113–132.
[5] Stone, J., L., J. D. Thomson and S. J. Dentacosta. 1995. Assessment of pollen viability in hand pollination experiments-a review. American J. Bot. 82: 1186-1197.
[6] Saini, H. S., M. Sedgley and D. Aspinall. 1984. Developmental anatomy in wheat of male sterility induced by heat stress, water deficit of absicisic acid. Australian J Plant Physiol. 11: 243-253.
[7] McLaren, N. W. and F. C. Wehner. 1992. Pre-flowering low temperature predisposition of sorghum to sugary disease (Claviceps africana L.). J. Phytopathology 135: 328-334.
[8] Sakata, T., H. Takahashi, I. Nishiyama and A. Higashitani. 2000. Effects of high temperature on the development of pollen mother cells and microspore in barley (Hordeum vulgare L.). J. Plant Research
113: 395-402.
[9] Ploschuk, E. L. and A. J. Hall. 1995. Capitulum position in sunflower affects grain temperature and duration of grain filling. Field Crops Research 44: 111-117.
[10] DeGrandi-Hoffman, G. and M. Chambers. 2006. Effects of honey bee (Hymenoptera: Apidae) foraging on seed set in self-fertile sunflowers (Helianthus annuus L.). Environmental Entomology 35: 1103-1108.
[11] Moriondo, M., C. Giannakopoulos and M. Bindi. 2011. Climate change impact assessment: the role of climate extremes in crop yield simulation. Climate Change 104: 679-701.
[12] Astiz, V. and L. F. Hernandez. 2013. Pollen production in sunflower (Helianthus annuus L.) is affected by air temperature and relative humidity during early reproductive growth. Int. J. Exp. Bot. 82: 297-302.
[13] Faegri, K. and J. Iversen. 1989. Textbook of Pollen Analysis. 4th ed. K. Fargri, P. E. Kalland and K.
Krzywinsk; J. Wiley and Sons, Chichester.
[14] Gupta, M. and Y. S. Murty. 1985. Viability and longevity of pollen in Vicia faba L. Acta Bot. Indica 13:
292-294.
[15] Thomson, J. D., Rigney, L. P., Karoly, K. M. and B. A. Thomson. 1994. Pollen viability, vigor and competitive ability in Erythronium grandiflorum (Liliaceae). American J. Bot. 81: 1257-1266.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2043
ISSN 2229-5518
[16] Wilson, M. F. and D. W. Schemske. 1980. Pollinator limitation, fruit production and floral display in paw
paw (Assimina triboba). Bull.torrey Bot. club 107: 401-408.
[17] Snow, A. A. 1982. Pollination intensity and potential seed set in passiflora vitifolia.oecologia. 55:231-237. [18] Gross, R. S. and P. A. Werner. 1983. Relationship among flowering phenology insect visitors and seed set
of individuals: experimental studies on four co-occuring species of golden rod (Solidago: compositae) Ecological Monographs. 53: 95-117.
[19] Schemske, D. W. and L. P. Pautler. 1984. The effect of pollen composition on fitness components in neo tropical herb. Oecologia. 62: 31-36.
[20] Weins, D. 1984. Ovule survivorship broad size, life history, breeding systems and reproductive success in plant. Oecologia. 64: 47-53.
[21] Zimmerman, M. and G. H. Pyke. 1988. Reproduction in polemonium: assessing the factors limiting seed set. American Nat. 131: 723-738.
[22] Lee, T. D. and F. A. Bazzaz. 1982. Regulation of fruit and seed production in an annual legume, Cassia fasciculate. Ecology. 63: 1363-1373.
[23] Norton, J. D. 1966. Testing of plum pollen viability with tetrazolium salts. Proceedings of the American
Society for Horticultural Sci. 89:132–1.
[24] Hauser, E. J. P. and J. H. Morrison.1964. The cytochemical reduction of nitro blue tetrazolium as an index of pollen viability. American J. Botany 51:748–752.
[25] Gul, H. and R. Ahmad. 2006. Effect of salinity on pollen viability of different canola (Brassica napus L.)
cultivars as reflected by the formation of fruits and seeds. Pakistan J. Bot. 38(2): 237-247.
[26] Steel, R. G. D., and J. H. Torrie. 1997. Principles and Procedures of Stastics. (2nd ed.) Mcgraw-Hill, New
York.
[27] Tukey, J. 1949. One degree of freedom for non-additivity. Biometrics 5(3): 232-242.
[28] Alexander, M. P. 1969. Differential staining of aborted and non-aborted pollen stain technology. 44:117
122.
[29] Godini, A. 1979. Counting pollen grains of some almond cultivars by means of an haemocytometer.
CIHEAM – options Mediterranean institute di Coltivazioni Arborees Universitodi Bari.
[30] Andrei, E. and G. Tridea.1998. Viability of sunflower pollen. Cercetari-Agronomice-in-Moldova Publ.
31(3-4): 121-126.
[31] Alkio, A. and E. Grimm. 2003. Vascular connections between the receptacle and empty achenes in sunflower (Helianthus annuus L.). Exp. Bot. 54: 345-348.
[32] Lindstrom, L. I., M. E. Garcia and L. F. Hernandez. 2004. Morphology and distribution of incompatibility developed fruits in sunflower (Helianthus annuus L.) capitula XVI Int. Sunflower conf. Procs. Fargo USA, ISA: 333-337.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 2044
ISSN 2229-5518
[33] Shahzad, M. A. and M. Rashid.2006. Determination of pollen viability and role of honey bees Apis Cerana
F. In the pollination of sunflower CMS lines in isolated tunnels. Pak. Entomol. 28(2): 69-72.
[34] Kumar, G. and P. Srivastava. 2009. Gibberellic acid-induced pollen mortality and abnormal microsporogenesis in safflower. Cytologia. 74: 171-176.
[35] Todorova, M., N. Nenova and J. Encheva. 2004. Study on in vitro pollen germination of sunflower
(Helianthus annuus L.) before and after gamma-radiation. Bulgariam Agric. Science 10: 65-70.
IJSER © 2015 http://www.ijser.org







