International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1046
ISSN 2229-5518
Effects of bath parameters on structure characteristic of Ni-W Electrodeposited alloy Farah A. Abed and Latef M. Ali
Hawler Technical Institute / Erbil – Iraq
—————————— ——————————
“Engineering” or “functional” coatings are broadly employed commercially to provide
enhanced surface performance for engineering components. Typically wear and corrosion resistance are the two critical properties for an engineering coatings, with secondary considerations including surface finish, coefficient of friction, surface coverage and throwing power [1].
Electrodeposition of alloys is particularly important for applications to microfabrication technologies [2] because of its low price, relatively simple technological equipment, low energy consumption, achievable high precision and mass production [3].
Tungsten with its unusual properties such as
highest melting point ( 3410 oC) of all metals,
lowest coefficient of linear thermal expansion (
4.3× 10−6 / oC) , highest tensile strength of ( 410
kg/mm2) and one of the highest young’s modulus
of elasticity ( 3500 kg/ mm2), can render excellent
properties to its alloy[4]. The presence of tungsten
in amorphous alloys increases the corrosion resistance of such alloys and it has good resistance of strong oxidizing acids at high temperatures and may compete even with ceramics and graphite by virtue of high thermal resistance [4, 5]. Nickel and tungsten are insoluble in the solid and liquid phase because of the high difference in diameter, higher than 15% [6]. For this reason, it is expected that a great variety of non-equilibrium (metastable) structural phases, and hence properties, to be
formed at alloying of these chemical elements [7]. In this paper the synthesis, structure and some of the properties of electrodeposited Ni-W alloy will be presented.
Graphite disc with diameter (D=3 cm) and thickness (d = 1cm) used as substrate (cathode) were chemically polished by 5% sulfric acid then washing by a distilled water and dried by a hot dry air. High purity platinum sheet was used as the anode and was placed (5cm) from cathode.
The Ni-W film were deposited from a plating
bath with citric aqueous bath chemistry : 20 g/l
Nickel sulfate 50 g/l of sodium tungstate, 66 g/l of
Nickel of citric acid and (5-10g/l) thiourea with a
PH fixed 7± 0.2 by adding of HR2RSOR4R or NaOH
solution and was controlled by means of the
electronically operated PH- meter . Deposition
was carried out with rotation at rates of (60-75 rpm) by magnetic stirring and at high temperatures (60-80 oC) for obtaining a high percentage of tungsten in the deposit. During alloy film preparation the plating cell was tightly capped in order to minimize the effect of air flow. The dc current density reached optimal values of (7-15
A/dm2). The film thickness h, currently ranging from (3-4 µ m) was measured by weightening method (h= w/ad) where h is the film thickness, w is the weight of the film, a is the area of the substrate and d is the thickness of substrate. The attached (EDS) energy disperse spectroscopy was used to determine the approximate composition of alloy,
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1047
ISSN 2229-5518
transmission electron microscopy (TEM) was employed to characterize the microstructure and
morphology of the specimen.
In this work, over 40 samples were coated under variable operating conditions (current density, temperature, stirring and bath additives
concentrations). Table (1) shows an interest samples here. The approximate composition of the
alloy was examined by EDS (Oxford Isis system). Optical microscopy (Olympus IX 71) was used to test the structure and morphology of deposits.
Table1.
Relation between current density, temperature and W% with stirring and without.
j A/dm2 | T oC | W% With stirring | W% Without stirring | T oC | W% With stirring | W% Without stirring | T oC | W% With stirring | W% Without stirring | Conc. of thi. g/l |
7 | 60 | 18 | 14.2 | 70 | 25.8 | 20.4 | 80 | 27.2 | 21.2 | 0 |
12 | 60 | 22.2 | 18 | 70 | 29.5 | 24.2 | 80 | 31 | 25 | 0 |
15 | 60 | 26.5 | 20 | 70 | 31.3 | 26.5 | 80 | 32 | 27.8 | 0 |
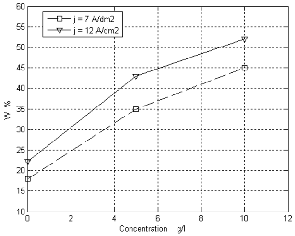
The plating variables effect on the composition of the deposit is investigated above. The effect of concentration of thiourea at (60 oC), PH (7) with stirring and current densities (7 and 12 A/dm2) on the W weight percent in the deposits is shown in figure 1.
Figure 1. Shows the relation of W (weight percent )in the deposit with concentration of thiourea at PH=7, (j= 7 and 12
A/dm2), T= 60 oC and with magnetic stirring.
The W weight percent in the deposit increases when the concentration of thiourea is increased. An increased in concentration of thiourea under given conditions increases the efficiency and cathodic polarization [8, 9]
and therby increases the deposition rate and the W
content in the deposit.
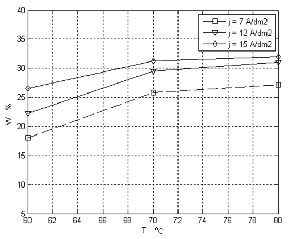
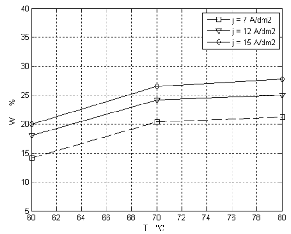
Temperature is an important factor in the plating process of Ni-W alloy .When the concentration of thiourea is (5g/l), both the W weight percent in plating bath with and without stirring is increased with increasing temperature of the bath. Figure (2, 3) shows the effect of temperature of different current densities on the tungsten content in two cases (with and without stirring).
The curves assume that the weight percent of W in the
alloy increases with increasing temperature .The
optimum temperature the cathode current efficiency high and the coating is smooth with fine grain size and bright [7-10]. It seems that the increase of temperature favors the W transport to the cathodic surface due to the increasing the mobility of ions and decreasing in the viscosity of the plating solution, so that the cathode film is more rapidly replenished, make its co- deposition easy [11,12].
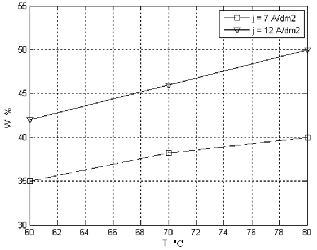
When the current density was increased, the W content
was increased linearly when vigorous stirring was
applied due to increase in the electrodeposition rate. But in the absence of stirring, a maximum was observed at 15 A/dm2. At high current densities, hydrogen evolution becomes more prominent and cause additional agitation in solution in addition to the possibility of deposition of Ni easily compared to W this effect was observed in figure (4a, b).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1048
ISSN 2229-5518
 a
a
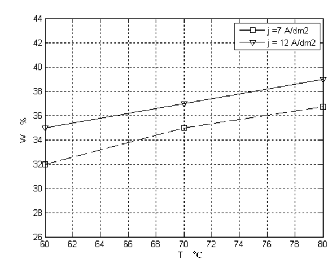
Figure 2. Describes the relation of temperature with W ( weight percent )at different current density with magnetic stirring in 5g/l thiourea.
b
Figure 4. The relation of W content with temperature at different values of current density with no additives a- with stirring and b- without stirring.
Figure 3. Shows the effect of the absence of magnetic stirring on the W (weight percent )at different temperature and current density with 5g/l thiourea.
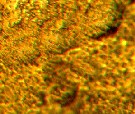
Figure (5 a, b) shows the effect of stirring on the Ni-W
deposit structure with 10 g/l thiourea, T= 60 oC and j
=7A/dm2.
a b
Figure 5. Shows the effect of stirring on the Ni-W deposit with 10 g/l thiourea, T= 60 oC and j =7A/dm2 a- with magnetic stirring and b- without stirring.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1049
ISSN 2229-5518
Magnetic stirring gives fine grain structure and
uniform morphology of the deposit with compare to absence of stirring. The cause of this behavior is the variation in the concentration of ions that directed towards the anode accompanied with hydrogen ions that affected on the structure and properties of the deposit [4, 13, and 14]
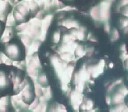
The addition of thiourea to deposit led to refining the structure and strong change in coating appearance [15], figure (6 a, b and c) shows that when the concentration of thiourea is 5 g/l the coating layer is bright with fine grained structure, while when the concentration of thiourea increased to 10 g/l, the deposit layer is characterized by finer grain structure with more bright appearance.
a b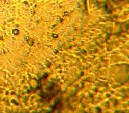
c
Figure 6. Shows the effect of thiourea concentrations on the grain structure of Ni-W alloy, a- no additives b- 5 g/l thiourea c- 10 g/l thiourea, T= 60 oC, j = 12 Adm2 and with magnetic stirring.
1- This research demonstrated the synergistic effect of the stirring of the bath, high current density and high bath temperature on the structure of Ni-W alloy.
2- Thiourea baths with different concentrations helps to refine the structure of the Ni-W alloy led to promote the mechanical properties of deposit.
3- The tungsten content in the alloy affected by the bath parameters (current density, stirring, additives and temperature).
4- As the bath temperature is increased, the tungsten content is increased in the presence of stirring and low change in W content with the absence of stirring.
5- At high current density, the tungsten content
increased linearly in the stirring bath but in the absence of stirring leads to maximum value of 15
A/dm2.
6- The grain size is inversely proportional to the thiourea concentration and it affects by bath parameters.
[1] B. Navinsek , P. Panjan and I. Milosev ,Surface and Coating
Technology, vol. 116 , pp. 476-487 (1999).
[2] R. Mousavi. K. Raeissi and A. Saatchi, International journal of Modern Physics B22, pp.3060 (2008).
[3] O.Younes, L. Zhu and E. Gileadi, Electrochem. Acta, vol.48, pp.2551-2256 (2003).
[4] Nasser Kanani , Electroplating; Basic principles, processes and practice Elsevier , Berlin , Germany 2004.
[5] T. Yamasaki, R. Tomohira, Y. Ogino , P. Schlobmacher and
K. Ehrlich , Plat. Surf. Finish., vol.87,pp.148-155, (2000).
[6] A. Brenner ,Electrodeposition of alloys : principle and practice ( American press Inc. Newyork, NY USA 1963).
[7] P. Datta, MS Thesis, Department of Mechanical
Engineering, Louisiana StateUniversity, (2001).
[8] N.Sulitanu , F. Brinza ,J. of optoelectronics and adv. Mat.
,vol. 5,no. 2 ,pp. 421-428, (2003).
[9] T. Osaka, Electrochem. Acta, 45 , 3311 ( 2000)
[10] B. Podgornik, Surface and Coating Technology, 146 (2001).
[11] Cesiulis , H. Podlaha – Murphy ,Mat. Sci., vol.9 ,no.4 , pp.324328, (2003).
[12] Cesiulis , H. Donten and M. L. Stojek, Mat. Sci. vol.
7,no. 4 , pp.237-244, ( 2001).
[13] Y. Tsuru, M.Nomura and F. R. Foulkes, J. Appl.
Electrochem. ,vol.32 ,pp.629-633, (2002)
[14] T. Jiang, N. Hall and S. Morin , Thin Solid Films, vol.76
,pp.471-478, (2005).
[15] I.Paseka, Electrochem. Acta,vol. 38,pp. 2449 2451,(1993).
IJSER © 2014 http://www.ijser.org