
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 681
ISSN 2229-5518
Effect of replacement coke breeze by charcoal on technical operation of iron ore sintering Naglaa.A.El-Hussiny(1), ,Ahmed.A. Khalifa (1), Ayman.A. El-Midany(2,3) , Ahmed.A.Ahmed (2),
Mohamed.E.H.Shalabi (1)*
Abstract— The coke breeze is the common solid fuel for iron sintering plants. The high consumption of coke breeze leads to its depletion as the most of the fossil fuels. Several researches, nowadays, focus on finding different alternatives that can replace the coke breeze either partially or completely. In this study, the charcoal was used as a supplementary fuel in the iron ore sintering process. Coke breeze was partially replaced by charcoal in sinter charge. The results of this work shows that the replacement of coke breeze with charcoal up to30% increased the vertical velocity of sintering process, strength of produced sinter, productivity of sinter machine and productivity of machine at blast furnace yard.
Index Terms— coke breeze, charcoal, sintering process, productivity of sinter machine
—————————— ——————————
RON production is an energy demanding process. Most of the heat consumed in the production of molten iron is typi- cally provided by the combustion of a solid fuel, i.e. coke. For example, in an integrated steel works the heat required for making sinter is usually provided by combustion of coke breeze. In some works the total amount of coke breeze re- quired for sintering cannot be met from this operation and additional fuel sources have to be sought[1].Widespread and massive consumption of fossil fuels has led to their depletion, increased carbon dioxide in the atmosphere and consequently
caused global warming and climate change[2-3].
On the other hand, biomass utilization as a source of energy is important from an energetic as well as an environmental viewpoint[4-5].It reduces the rate of fossil fuel depletion and it has the theoretical potential to supply100% of the world's en- ergy needs[3].
The use of solid biomass as an alternative fuel in the process of production of ironore agglomerate is determined by its chem- ical and physical properties[6]. JozefHudák et al[7] observed that charcoal is a suitable substitute fuel in agglomeration pro- cess. In the technical and economic indicators, it is suitable to replace fossil fuels up to 44%.
Mohamed [8] indicated that partial replacement of coke breeze used as a fuel in iron ore sintering by palmstone charcoal could be achieved without deteriorating the sintering process or
————————————————
• (1)Central Metallurgical Research and Development Institute
(CMRDI), Cairo, Egypt.
• (2)Cairo University, Mining, Petroleum and Metallurgical Engineer-
ing Dept., Egypt.
• (3)Missouri University of Science & Technology, Mining and Nuclear
Engineering Dept., USA.
• * Corresponding author (shalabimoh@hotmail.com)
decreasing the metallurgical properties of produced sinters; when about one third of the coke breeze was replaced by char- coal.
Shalabi et al [9] indicated that when coke breeze was partial- ly replaced by 1.5 equivalent amount of palm stone energy- wise, the amount of ready-made sinter, productivity of sintering machine and productivity of blast furnace yard increased by
7.8%, 8.3% and 12.7%, respectively. It is appropriate to replace coke breeze by palm stone up to 15%.
Liming et al[10] illustrated that the sintering speed and productivity were maintained as the charcoal substitution in- creased to 25% and then increased considerably with further increase in the charcoal substitution until 50%. Ooi et al[11] suggested that it is feasible to substitute 10% coke breeze by biomass in the iron ore sintering process. In another work, Ooi et al[12]found that fuel blends where 20% of the coke breeze was replaced by charcoal may improve both the sinter yield and productivity. Similarly Gan et al[13] found that the appropriate proportion of charcoal, charred-straw and molded sawdust is
40%, 20% and 15% respectively.
CSIRO Minerals Iron Ore Processing Group has demonstrat-
ed that the coke breeze can be substituted by red gum char for iron ore sintering. It was found that adding 44% charcoal not only improves the productivity of sintering but also reduces the emissions of SORXR and NORXR[14].
The main object of this work is devoted to study the effect of replacing coke breeze with charcoal in the sintering process per- formance of El-Bahariya iron ore and the quality of produced sinter.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 682
ISSN 2229-5518
The raw materials used in this work are iron ore concentrate from El-Bahariya high barite ore, limestone, coke breeze and charcoal. Their chemical analyses are shown in Table 1. In ad- dition, the physico-chemical characteristics of charcoal and coke breeze are listed in Table 2.
.
TABLE 2
PHYSICO-CHEMICAL CHARACTERISTICS OF FUEL
TABLE 1
CHEMICAL ANALYSIS OF RAW MATERIAL FOR SINTERING PROCESS
The X-ray diffraction analysis of iron concentrate, coke breeze and charcoal are shown in Figures 1-3.From figure 1, it is clear that the Bahariya iron concentrate mainly consists of hematite, goethite and quartz, while coke breeze mainly consists of graphite and quartz as shown in Figure 2 and the charcoal fuel has amorphous structure as illustrated in Figure 3.
.
V.M.: volatile matter, F.C : fixed carbon

Fig.1. X-ray diffraction pattern of iron concentrate sample
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 683
ISSN 2229-5518

flame for a period of 2 min. The ignition was done under suc- tion pressure of 5.88 kPa, while the sintering process was done under suction pressure of 11.76 kPa. The sintering time was determined by the time elapsed from the start of ignition until the exhaust gas temperature reached the maximum value. At the end of the sintering experiment, the produced sinter was dropped on to a steel plate laid on concrete and then the sinter cake was screened over a +7 mm sieve, to determine the productivity of the sintering machine (P), as shown in Eq.
1[15].
Fig.2. X-ray diffraction pattern of the coke sample

Fig.3. X-ray diffraction pattern of charcoal sample
Sintering experiments were conducted in a laboratory down draft sinter pot (its diameter and height were 125 and
340 mm respectively, with capacity of 5 kg). Air flow was provided by two fans in series which were capable of produc- ing a suction pressure in excess of 12 kPa. Pt-Pt. Rh. Thermo- couple measured the temperature of the waste gas, which gives an indication of the end of the sintering process. The represented iron ore, limestone, 30% of the charge sinter re- turn, coke breeze (1-3mm) and charcoal were blended together and then water (12% of the charge) was added through mixing the raw materials. The sinter charge basicity (CaO/SiO2= 0.9, was kept constant throughout all experiments of the influence of the replaced coke breeze with charcoal.
The green charge was loaded over the sinter bed layer 0.5 kg sinter (+ 10 mm). The green raw mix was ignited with a gas
P = 14.4*K*ρ*V ton/m2day (1) Where:
K is the percentage of ready-made sinter from the charge.
ρ is bulk density of the charge, ton/m3.
V is vertical velocity of sintering machine
(V = H/T) m/min.
H is the height of the charge in sinter pot, mm. T is the time of sintering, min.
The sinter cake above 7 mm was dropped four times from a height of 2 m shatter test to measure the sinter strength. Sin- ter strength (Ss) as illustrated in Eq. 2, was calculated as the percentage of sinter (above 7 mm) after shatter test, and relat- ed to the ready-made sinter strength[16].
Ss % = W1/W2 * 100 (2) Where Ss% is the sinter strength percentage, W1, W2 repre-
sent the weight of sinter (above 7 mm) after and before shatter test, respectively.
The productivity of the sinter machine at blast furnace yard
(PB.F) was calculated according to the following equation[16]:
PB.F = p * Ss (3) Where PB.F is the productivity of the sinter machine at
blast furnace yard, expressed in ton/m2 per day, P is the productivity of sintering machine, also expressed in ton/m2 per day and Ss% is shatter index (sinter strength ) above 7 mm.
The reducibility of the produced sinter was done by hydro- gen in thermo-gravimetric apparatus at temperature 800 oC, flow rate of H2 = 1.5 l/min and 60 min reduction time. The schematic diagram of the reduction apparatus is given in Fig- ure 4 and its details are present elsewhere[17].. The reducibil- ity degree was calculated by measuring the weight loss of the produced sinter as shown in equation number 4.
Percent of reduction = [(W0 –Wt) ×100/ Oxygen mass (4)
Where:
Wo: the initial mass of produced sinter sample, g.
Wt: weight of sample after each time t.
Oxygen mass: indicates the mass of oxygen percent in
sinter in the form of FeO and Fe2O3, g.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 684
ISSN 2229-5518
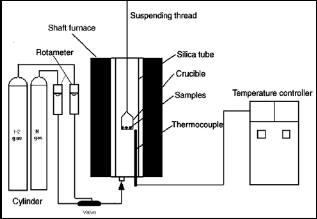
Figure 4. Schematic diagram of the reduction apparatus
.
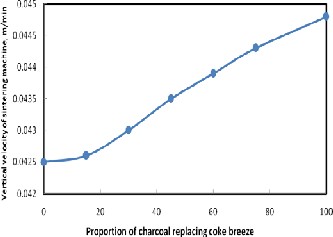
The effect of the proportion of charcoal replacing coke breeze on the vertical velocity of sintering process is shown in Figure 5. It is indicated that the vertical velocity of sintering process increases with increasing the proportion of charcoal in the charge. This may be attributed to the faster burning of charcoal as a result of its high porosity and large surface area in comparison to coke breeze[13].
3.1.2. EFFECT OF REPLACEMENT OF COKE BREEZE BY CHARCOAL ON SINTER STRENGTH AND READY-MADE SINTER.
Figure 6 illustrates the effect of replacement of coke breeze with charcoal on the amount of produced ready-made sinter and its strength. It is clear that as the amount of charcoal re- placement increases, the ready-made sinter and its strength increased up to 30 % charcoal. Increasing the charcoal above
30% negatively affect the strength and ready-made sinter. This is due to the fast combustion of charcoal rather than coke breeze[13].
Figure 5. Effect of the proportion of charcoal replacing coke breeze on the vertical velocity on the sintering process.
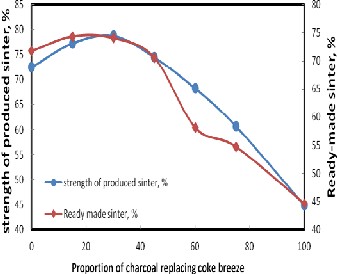
Figure 6. Effect of the proportion of charcoal replacing coke breeze on the strength and the amount of ready-made sinter.
Figure 7 shows the relation between the proportion of char- coal replacing coke breeze and both the productivity of sinter- ing machine and the productivity at blast furnace yard. It is clear that the optimum productivity of the sintering machine
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 685
ISSN 2229-5518
and the productivity at blast furnace yard were attained at
30% coke breeze replacement by charcoal in the sinter raw mix
and was about 58.51% and 46.11%, respectively. This due to
decrease in sintering time suggests that replacement of coke
with charcoal may lead to an increase in sinter productivity if
a reasonable sinter yield is maintained[12]. The change in the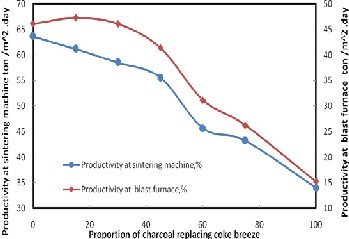
productivity of sintering machine and productivity at B.F.yard is due to the combination of ready-made sinter, tine of sinter- ing and the strength of the produced sinter.

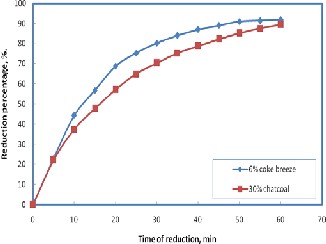
Figure 8. Effect of replacement of coke breeze by charcoal on the re- duction percentage
Figure 7. Effect of the proportion of charcoal replacing coke breeze on the productivity of sintering machine and the productivity of B.F yard
Reduction was carried out at temperature 800 °C, where the initial weight of sinters were fixed at the same value (10 gm) and the hydrogen flow rate =1.5 liter /min. The results are illustrated in Figure 8 for sinter used 100% coke breeze and
30% coke breeze replaced by charcoal. It is clear that the re- duction of sinter produced by 30% coke breeze replaced by charcoal is higher than the sinter used 100% coke breeze this is may be due to the porosity of the produced sinter of 30% coke breeze replaced by charcoal is higher than sinter produced by
100% coke breeze, Figure 9.
A) B)
Figure 9 The microscopic structure of iron ore sinter (A) sample of the sinter used 6% coke breeze (B) sample of the sinter used 70% coke breeze + 30% charcoal (black = pore, white and gray = magnetite and wustite embedded in silicate matrix).
The depletion of the fossil fuels such as coke breeze requires searching for other alternatives that maintain the performance of the iron sintering process. Charcoal is one of these alterna- tives that applied in the current study as a supplementary ma- terial for coke breeze. A gradual increase in the charcoal por- tion replacement was tested until it reaches 100% charcoal. However, the results show that the optimum portion of char- coal is 30%. Replacement of coke breeze up to 30% by charcoal in iron-ore sinter charge resulted in increasing the vertical ve- locity of sintering process, increasing the ready-made sinter and its strength and increasing the reducibility of sinter by hydrogen at 800 °C. Moreover, at 30% charcoal, the optimum of productivity of sintering machine and the productivity at B.F. yard is about 58.51% and 46.11%, respectively.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 686
ISSN 2229-5518
[1] [1] M. Zandi, M. Martinez-Pacheco, and T. A. T. Fray, “Biomass for iron ore sintering,” Miner. Eng., vol. 23, no. 14, pp. 1139–1145, Nov.
2010.
[2] [2] F. Vojdani, “Biomass and waste characterization as a potential energy sources in Iran,” in Proceedings of the third international symposium on energy from biomass and waste, 2010, pp. 8–11.
[3] [3] M. A. Youssef, S. S. Wahid, M. A. Mohamed, and A. A.
Askalany, “Experimental study on Egyptian biomass combustion in circulating fluidized bed,” Appl. Energy, vol. 86, no. 12, pp. 2644–
2650, Dec. 2009.
[4] [4] Z.-A. Sun, B.-S. Jin, M.-Y. Zhang, R.-P. Liu, and Y. Zhang, “Ex- perimental study on cotton stalk combustion in a circulating fluid- ized bed,” Appl. Energy, vol. 85, no. 11, pp. 1027–1040, Nov. 2008.
[5] [5] H. M. El-mashad, W. K. P. Van Loon, G. Zeeman, and G. P. A.
Bot, “Reuse potential of agricultural wastes in semi-arid regions :
Egypt as a case study,” no. Iea 2000, pp. 53–66, 2010.
[6] [6] M. Fröhlichová, J. Legemza, R. Findorák, and a. Mašlejová, “Bi- omass as a Source of Energy in Iron Ore Agglomerate Production Process,” Arch. Metall. Mater., vol. 59, no. 2, Jan. 2014.
[7] [7] J. . Hudák, M. Butkovská, and J. Leško, “EFFECT OFBIO- MASSAS AREPLACEMENTFUEL ON IRON ORE AGGLOMERATE QUALITY,” vol. 4, pp. 47–55, 2014.
[8] [8] O. A. Mohamed, “The feasibility study of palm stone charcoal as a fuel in sintering iron ore,” in in proccedings sixth international symposium on Agglomeration, 1993, pp. 208–212.
[9] [9] M. E. H. Shalabi, O. A. Mohamed, and N. A. El-Hussiny, “The use of palmstone charcoal in the sintering of Baharia iron ore,” in The fifth international conference of chemical Engineering, 1996, pp. 109–
121.
[10] [10] L. Lu, M. Adam, M. Kilburn, S. Hapugoda, M. Somerville, S.
Jahanshahi, and J. G. Mathieson, “Substitution of Charcoal for Coke
Breeze in Iron Ore Sintering,” ISIJ Int., vol. 53, no. 9, pp. 1607–1616,
2013.
[11] [11] T. C. Ooi, E. Aries, B. C. R. Ewan, D. Thompson, D. R. Ander- son, R. Fisher, T. Fray, and D. Tognarelli, “The study of sunflower seed husks as a fuel in the iron ore sintering process,” Miner. Eng., vol. 21, no. 2, pp. 167–177, Jan. 2008.
[12] [12] T. C. Ooi, D. Thompson, D. R. Anderson, R. Fisher, T. Fray, and M. Zandi, “The effect of charcoal combustion on iron-ore sintering performance and emission of persistent organic pollutants,” Com- bust. Flame, vol. 158, no. 5, pp. 979–987, May 2011.
[13] [13] M. Gan, X. Fan, X. Chen, Z. Ji, W. Lv, Y. Wang, Z. Yu, and T.
Jiang, “Reduction of Pollutant Emission in Iron Ore Sintering Process by Applying Biomass Fuels,” ISIJ Int., vol. 52, no. 9, pp. 1574–1578,
2012.
[14] [14] M. Dell Amico, P. Fung, R. Lover, J. Manuel, and M. O Conner, “Green Iron OreSintering,” in proc. con. Green processing 2004.
[15] [15] E. F. Vegman and B. N. Jereben, “Metallurgy of Pig Iron,”
metallorgy, 1978.
[16] [16] N. A. El-Hussiny, F. M. Mohamed, and M. E. H. Shalabi, “Recy- cling of mill scale in sintering process,” Sci. Sinter., vol. 43, no. 1, pp.
21–31, 2011.
[17] [17] N. A. El-Hussiny and M. E. H. Shalabi, “A self-reduced inter- mediate product from iron and steel plants waste materials using a briquetting process,” Powder Technol., vol. 205, no. 1–3, pp. 217–223,
Jan. 2011.
IJSER © 2015 http://www.ijser.org