𝑆. 𝐹. 𝐶 =
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1005
ISSN 2229-5518
Effect of Starch and Gum Arabic Binders in the
Combustion Characteristics of Briquette
Prepared from Sawdust
Department of Mechanical Engineering, Federal University of Technology, Minna, Nigeria
Keywords— Binders, Briquette, Sawdust, Starch, Gum Arabic.
—————————— ——————————
NDUSTRIALIZATION has made energy absolutely essential to manufacturing, commerce, transportation, comfort, agri- culture, lighting and our daily activities. Importance of energy differs for different social-economic setting. For developed nations, energy abundance may mean difference between eco- nomic growth and a period of economic decline in a country and possibly, a shift in lifestyles from energy extravagance to moderation. High-energy consumption has been associated with higher quality of life, which in turn is related to Gross National Product (GNP). Economic development amongst nations has drawn interest to global energy resource invento- ries as well as regional or country wise energy source endow- ments. Source of energy can be grouped into renewable and non-renewable. Renewable energy is infinite energy source obtained from the continuing or repetitive current of energy occurring in natural environment [1]. These sources include biomass, solar energy and wind energy. While, non-renewable energy is one, which is finite, exhaustible and cannot be re- placed. These are type of energy obtained from static stores of energy that remain bound unless released by human interven- tions. Non-renewable energies such as fossil fuel, petroleum, coal, natural gases and nuclear fuels are some of the several
available to man [2].
Due to oil crises, progress has been made considerably to-
wards finding new sources of fuel under secure Western con-
trol, increasing diversity of supply and widely spread reserves
of natural gas, which rapidly displaced oil in much non- transport uses. Nearly all the western world’s energy still comes from fossil fuel that will become increasingly hard to secure. Affordable oil and gas are unlikely to last more than another century. As supplies reduces, prices will rise and use will increasingly be restricted to premium applications like in transport and plastics manufacture shortage will create a re- newed risk of instability in international markets. Nigeria as a member of OPEC, which she joined in 1971, has been firstly a beneficiary and secondly, a victim of the price increases [3].
Wood was the first the major source of energy and was readily available, because extensive forests grew in many parts of the
world and the amount of wood needed for heating and cook- ing was relatively modest. However, the situation changed when wood began to be used during the middle ages to make charcoal. The climate in many developing nations like Nigeria favours renewable energy, if only there is commitment on the part of government and stakeholders in energy matters in Ni- geria to indicate or initiate major policies that would be sup- ported by a variety of other measure including targets, energy studies and strategies. One of such alternative source of ener- gy is biomass energy, which is highly favoured because of its result in cleaning environment. Briquetting this biomass for energy source utilization could also be the solution to the ever increasing energy crises and shortage of energy in the country.
Briquette is the physic-mechanical conversion with or without an additive of dry loose sawdust of fine particles size into sol- id state characterized by a regular shape and high density [4]. These processes could also be defined as process of converting loose wastes or sawdust into a dense, compact and consolidat- ed unit through the application of high pressure. Apart from the problems of transportation, storage and handling, the di- rect burning of sawdust in conventional grates and stove are associated with very low thermal efficiencies and widespread of air pollution. In addition, a large percentage of un-burnt carbonaceous ash has to be disposed. Briquette of this waste could militate against these pollution problems while at the same time making use of this important industrial/domestic energy source. The briquettes produced are normally of two forms: carbonized and non-carbonized, carbonized briquettes are formed when biomass is subjected to a destructive distilla- tion in a large closed reactor devoid of oxygen and quench after attaining a predetermined temperature. On the other
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1006
ISSN 2229-5518
side, non-carbonized briquettes are plain briquettes produced without undergoing carbonization process.
𝑆. 𝐹. 𝐶 =![]()
𝑚𝑓
𝑚𝑤
(3)
In Nigeria, a lot of research work had been done on the uti- lization of sawdust for domestic cooking using improved burning stone [5-9] while attempt had been made to densify the sawdust with appropriate equipment fabricated by the raw materials Research and Development Council in collabo- ration with Abubakar Tafawa Balewa University, Bauchi [10]. There has been various research works at the Federal Univer-
Where: 𝑚𝑤 is mass of water in the pot (kg); 𝑚𝑓 is Mass of fuel
burnt (kg).
The burning rate is the rate at which a certain mass of fuel is combusted in air. It is given as:
sity of Technologies, in both Minna and Akure on the suitabil- ity of utilizing briquettes fuel as high-grade solid fuel [11-15]. This involved the use of common sawdust with pat kernel
𝑚
![]()
𝐵. 𝑅. =
𝑡
(4)
shell adds line to improve calorific value. Water boiling test will be used to simply simulate the standard cooking proce- dures. Therefore, the current research is focused utilization of sawdust biomass for domestic supply of energy. It investigates the effect of starch and gum arabic binders in the combustion characteristics of briquette prepared from sawdust and prop- erties such as calorific value (CV), volatile matter, flame tem- perature, percentage heat utilized (PHU), specific fuel con- sumption (SFC) and time spent in boiling were determined to evaluate its performance.
The calorific value of a fuel is the amount of energy liberated by burning a unit mass of the fuel. The calorific value of the biomass briquette is expressed as;
𝑃𝑓 ×𝑡
Where: 𝑚 is Mass of fuel burnt (kg) and t is the time (s).
Sawdust sample used in this research was collected from timber market, Minna and divided into three different portions. The first sample contains raw sawdust without briquetting medium. The se- cond sample is a measured quantity of sawdust briquetted using starch and the third sample is an equal quantity of sawdust with gum arabic as the briquetting medium. Prior to its separation into three parts, the sawdust (biomass) was screened of impurities like sand, metallic objects and chips of wood. It was sun dried to reduce mois- ture content and sieved using 2mm sieve to ensure uniform grain size and ease of compression. The ratio employed in this study was based on previous work of [12]. Other equipment used in this study in- clude; starch paste, gum Arabic, water, weighing balance, briquette machine, briquette stove (Figure 1(a)), cooking pot, bomb calorime- ter and crucible.
The starch paste and gum Arabic were made into non-viscous gel
𝐸𝑓 =
𝑚𝑓
![]()
(1)
preparatory to briquette. The samples were mixed at different propor- tion. Starch and gum Arabic, which is a binder, was also added to
Where: 𝐸𝑓 is calorific values of the fuel (KJ/Kg); 𝑚𝑓 is mass of fuel burnt (kg); 𝑃𝑓 is power output of the fuel (W); t is the time
(s).
enhance binding force. A manually operated hydraulic Briquette machine designed and fabricated in the Federal University of Tech- nology, Minna was used for the compression of the samples into cylindrical shaped briquettes. The machine is equipped with pressure gauge, a hand lever, with a mould capable of producing 15 briquettes at the same time. Briquettes of the various mixtures were made under
2
Percentage Heat Utilized (P.H.U) is the ratio of the net heat
maximum pressure of 110kN/m
to enhance perfect compassion. The
supplied to the water and the net heat liberated by the fuel. It is given as;
𝑚𝑤 𝐶𝑝 (𝑇𝑏−𝑇𝑖)+𝑚𝑒𝐿
pressure was then released gradually to eject the compressed bri-
quettes. After each successive operation whereby cylindrical bri-
quettes were obtained, the products were sun dried in open space as shown in Figure 1(b) to remove the inherent moisture content to a
𝑃. 𝐻. 𝑈 =
𝑚𝑓 ×𝐸𝑓
![]()
× 100 (2)
tolerable level.
Where: 𝑚𝑤 is mass of water in the pot (kg); 𝑚𝑒 mass of water evaporated (kg); 𝑚𝑓 is mass of fuel burnt (kg); Ti is Initial
temperature of water (K); Tb is boiling temperature of water
(K); cp is Specific heat of water (KJ/KgK); L is Latent heat of
temperature (KJ/Kg); 𝐸𝑓 is calorific values of the fuel (KJ/Kg).
The Specific Fuel Consumption (S.F.C) is the ratio of the mass biomass briquette burnt to the mass of water boiled. It is given as;
Figure 1: Materials; (a)-Cylindrical briquettes, (b)-Briquette stove
Water boiling test (WBT) which has been used by other researchers
[13] was used in this experiment. In conducting water-boiling test,
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1007
ISSN 2229-5518
the following procedures were taken. Dry weight of experiment ma-
terials like pot and stove were taken and recorded. The pot was filled with 1kg of water. The same mass (1kg of water), was maintained at each succession test and throughout the course of carrying out a wa- ter boiling test. The initial temperature of water was taken. The ini- tial mass of briquettes fuel was taken before it was arranged in the briquette stove. A minimum of fifteen pieces of briquettes was used during each experiment. The fuel was then ignited by adding a little kerosene of (30g) to speed up the rate of initial combustion. It has been established [16] that 1g of kerosene is assumed to be about 2g of the briquettes and hence the kerosene used can be assumed to be part of briquette utilized. After the briquettes have been lit the pot containing 1kg of water was placed on the briquette stove and proce- dures continue. The temperature of the water was taken at 5 minutes interval until boiling took place. The time and the point of boiling was recorded and tagged “boiling phase”. The mass of fuel remain- ing inside the stove at this point was also recorded on the analogue
weighing balance. The pot of water was quickly transferred back to the stove and the process continued for the next 15 minutes after, which the above procedure was repeated, this point being tagged “high boiling phase”. At the end of the low boiling phase, the mass of water and fuel remaining were recorded. The mass of charcoal and ash remaining were recorded.
To perform the flame temperature test, 5g of the samples was weighed into a silica crucible and transferred into muffle furnace, the
temperature of the furnace was increased at a rate of 20⁰C energy
fine minute until active burning or flaming of the sample occurred.
The temperature of ignition was then recorded. Performing the Vola- tile Matter, 2g of moisture free samples were weighed into tarred, covered with the lid, the crucible and its content placed in the muffle furnace. It was removed after exactly 15 minutes residence in the hot zone of the furnace just before attaining the ignition temperature and was then cooled in a desiccator. The crucible with its content was weighed and expressed as the weight loss % volatile matter. The values obtained were used for analysis. The whole process was re- peated for all the samples of briquettes of different ratio of the bio- mass. From the data, the calorific values, Percentage Heat Utilized (PHU), Specific Fuel Consumption (SFC), flame temperature and time spent in boiling was calculated using equations (1) to (4).
The results of various tests investigated in the current research for calorific values, Percentage Heat Utilized (PHU), Specific Fuel Con- sumption (SFC), flame temperature and time spent in boiling are presented in Figures 2 to 7.
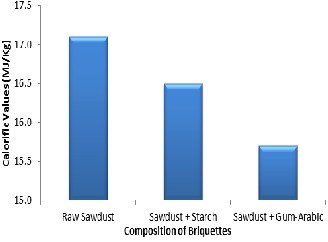
The calorific values of raw sawdust, sawdust briquette starch and sawdust briquette with gum-arabic are shown Figure 2. The results show that the non-briquette sawdust has a calorific value of 17.1
MJ/kg and will serve as a base for the comparison of the calorific values of the binders used. Figure 2 shows that the sample using starch as binder has a calorific value of 16.50MJ/kg, while the bri- quette using gum-arabic as binder has a calorific value of 15.7MJ/kg. These values show that using starch as a binder for briquette fuel has better performance than gum Arabic.
Figure 2: Calorific values of Briquettes
Figures 3 and 4 show that the non-briquette sawdust has a flame temperature and percentage volatile matter of 380oC and 69% re- spectively. It was observed that the sample with higher calorific val- ue has a higher flame temperature and vice versa. It was also noted that factors such as the plantation age and the moisture content of the
residue could affect the calorific value and flame temperature of a particular biomass. This could be confirmed by the values of per- centage volatile matter in each sample. The sample with the highest flame temperature of 380oC has 69% of volatile matter. Figure 3 and
4 show that the sample using starch as binder has a flame tempera- ture of 340oC and a volatile matter of 62%, while the briquette using gum-arabic as binder has a flame temperature 290oC and a volatile matter of 53%.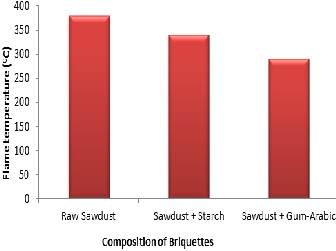
Figure 3: Flame temperature
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1008
ISSN 2229-5518
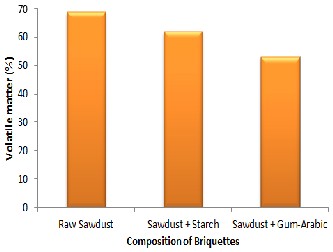
Figure 4: Percentage Volatile matter
The percentage heat utilized (PHU) during water test for raw saw- dust, starch binder and gum-arabic binder are 39.7%, 33.06% and
28.23% respectively. Thus, the fuel with a higher PHU and high car-
bon content is a better fuel as shown in Figure 5.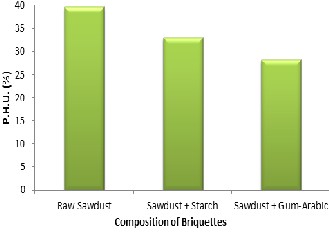
Figure 5: Percentage Heat Utilized
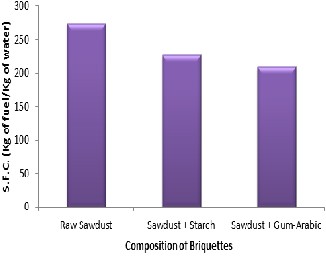
The specific fuel consumption (SFC) during water test for raw saw- dust, starch binder briquette and gum-arabic binder briquette are
274.5KJ/kg, 227.3KJ/kg and 208.5KJ/kg respectively as shown in Figure 6. It implies that the raw sawdust could be used for rapid cooking while the others could be utilized for both low and high cooking particularly in bakery where there is need for heat retention in the oven.
Figure 6: Specific Fuel Consumption
The time spent in boiling (TSB) 1kg of water using raw sawdust, starch binder briquette and gum-arabic binder briquette are 23mins,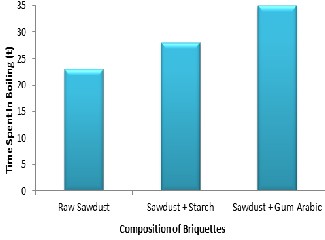
28mins and 35mins respectively as shown in Figure 7. The observed results may be due to a mixture of the biomass and the briquetting additives and also the presence of pores within the sawdust grains which might have been increased due to the addition of the briquette. The pores holding air or moisture have the effect of facilitating the escape of volatile gases and the breakdown of the structure as the briquette is burnt.
Figure 7: Time Spent In Boiling
The effects of binders on the combustion characteristics of sawdust (waste) have been investigated. Starch paste and gum Arabic was used to agglomerate the sawdust mixture together and utilized for high-grade solid fuel. The results obtained for calorific values, per- centage volatile content, flame temperature, percentage heat utilized, specific fuel consumption and time taken in boiling showed that starch is has a better performance as a binder than gum arabic. Alt- hough, it was revealed that the calorific values of the briquettes of different hard wood species involved in this study did not vary great- ly, marginal increment was observed ranging from 16.5–17.1 MJ/kg
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1009
ISSN 2229-5518
while the flame temperature varied greatly between 340oC and
380oC.Thus, it can be said that raw sawdust could be used for rapid cooking in households while others could be utilized for both low
and high rapid cooking particularly in bakery where there is need for heat retention in the oven.
[1]. Twidell, J.W. and Weir, A.D. (2006). Renewable Energy
Resources, Taylor & Francis, London and New York.
[2]. Adegoke, C.O. (1999). A Preliminary investigation of sawdust as high grade solid Fuel, Nigeria Journal of Re-
newable Energy.
[3]. Ayodele, A.S. and Nweke, G.A. (1987). Energy Devel- opment and Utilization in Nigeria. Energy Primary Re- source – the World and Nigeria, NISER, Ibadan.
[4]. Bamoyi, F.A. (1999). Biomass Energy for the Next Mil- lennium. A paper Delivered at Nigerian Association of
Small Scale Industrialist (NASSI) Lagos state branch.
[5]. Danshehu, B.G., Sambo, A.S., and Musa, M. (1992).
Comparative performance of sawdust and wood-burning
stoves. Nigerian J. Renewable Energy 3(1&2): 50-55.
[6]. Emerhi, E. A. (2011). Physical and combustion properties of briquettes produced from sawdust of three hardwood
species and different organic binders, Adv. Appl. Sci.
Res., 2(6):236-246.
[7]. Ismaila A., Zakari I. Y., Nasiru R., Tijjani B. I., Abdullahi
I., and Garba N. N. (2013). Investigation on biomass bri- quettes as energy source in relation to their calorific val- ues and measurement of their total carbon and elemental
contents for efficient biofuel utilization, Adv. Appl. Sci. Res., 4(4):303-309.
[8]. Oumarou M.B. and Oluwole F.A. (2010). Design of a Pe- dal Operated Briquette Press, Continental J. Engineering
Sciences 5:61 – 67.
[9]. Grover P. D. & Mishra S. K. (1996) Biomass briquetting: Technology and Practices, Food and Agriculture Organi- zation of the United Nations Bangkok, April 1996, Field Document No.46
[10]. Aliyu, A.A. (1996). Technology Development in Nigeria
Raw Materials Research and Development council, Abuja.
[11]. Kuti, O.A. (2003). Evaluation of composite sawdust bri- quette as a high grade fuel for domestic cooking. M.Eng Thesis, Federal University of Technology, Akure, Nigeria.
[12]. Adegoke, C.O. and Muhammad, T.I (2002). Investigation of Sawdust Briquette as High Grade Fuel, West Indian
journal of Engineering.
[13]. Kuti, O. A. (2009). Performance of Composite Sawdust Briquette Fuel in a Biomass Stove under Simulated Condi- tion, AU J.T. 12(4): 284-288.
[14]. Kuti, O.A. (2007). Impact of charred palm kernel shell on the calorific value of composite sawdust briquette. J. Engin. Appl. Sci. 2(1): 62-65.
[15]. Kuti, O.A.; and Adegoke, C.O. (2008). Comparative per- formance of composite sawdust briquette with kerosene fuel under domestic cooking conditions. AU J.T. 12: 57-
61.
[16]. Stewart, W. (1987). Improved Wood, Waste and Charcoal Burning Stoves. A Practical Manual. Intermediate Tech- nology Publications, Covent Garden, London, UK.
IJSER © 2014 http://www.ijser.org