
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 965
ISSN 2229-5518
Effect of Emitted Radiation from Mobile Phones and its Base Station Antennas on Some
Biochemical Parameters in Human Red Blood
Cells
Hathama Razooki Hasan *, Ali Haneen Issmer ** †
Abstract — The effects of radiations used in global system for mobile communications (GSM) on humans are an emerging area of investigation. Therefore, the purpose of this work is to study the effect of microwave/radiofrequency emitted from mobile phones and its base stations antennas on human health. The present study included measurement of total protein concentration, superoxide dismutase (SOD) activity, nitric oxide (NO•), and peroxynitrite (ONOO-) concentrations in human red blood cells (RBCs) samples that were collected from 87 healthy volunteers classified into three groups: Group T (36 volunteers) who reside nearby mobile phone base-station antennas. Group M (23 volunteers) were using commercially available mobile phone heavily, and the third group was the control group (28 volunteers) who reside faraway from base- station and had using mobile phone for less, or equal to 10 minutes daily. The results indicated presence of a highly significant increase in total protein concentration, SOD activity, NO• concentration and ONOO- concentration (P <
0.05).W hile the results indicated non-significant differences in the protein concentration and SOD activity in RBCs samples of group M in comparison with that of control group, while a highly significant increase in both NO• and ONOO- concentrations (P < 0.05). The results proved that the long exposure to emitted radiations from base stations and heavy using of mobile phone at domestic level has negative impact on human health due to increase the concentration of peroxynitrite, which can in turn cause many diseases like cancer.
The effects of radiations used in global system for mobile communications (GSM) on humans are an emerging area of investigation. Two systems of digital mobile phone commonly used, GSM 900 MHz and GSM 1800 MHz [1]. Except that radiation from base station antennas is about 100 times more powerful and uses a little higher carrier frequency, almost there is no deference between radiation from base station antennas and that from mobile phone of the same system (GSM 900 or GSM
1800 MHz) [2]. Radiofrequency (RF)-microwave radiations emitted from mobile phone has certain well-defined frequencies, which facilitate its discernment by a living organism, and via which the organism can, in turn, be affected by oscillatory electrical processes of various kinds, each characterized by a specific frequency, some of which happen to be close to those used in GSM. Thus some endogenous biological electrical activities can be interfered with via oscillatory aspects of the
————————————————
PH- 009647710003206.
E-mail: hathamahasan@scbaghdad.com
E-mail: alihaneen77@gmail.com
† Corresponding author
Abbreviations— CAT: catalase; DNA: deoxyribonucleic acid, ELF: extreme low frequency; EMF: electromagnetic field; GSM: global system for mobile communications; MHz: mega Hertz; NO•: nitric oxide, NOS: nitric oxide synthase; RBCs: red blood cells; RF: radiofrequency; RNS: reactive nitrogen species; ROS: reactive oxygen species; SOD: superoxide dismutase; UV: ultra violet.
incoming radiation, in much the same way as can the reception on a radio [3].Free radicals are a group of highly chemically reactive, short-lived molecules consisting of unpaired electrons in the outer orbital [4, 5]. These free radicals are included reactive oxygen species (ROS) and reactive nitrogen species (RNS) [6]. Increased free radicals formation together with a reduced antioxidant defense known oxidative stress [7]. Mobile phones radiation may affect the biological systems by increasing free radicals that enhance lipid peroxidation, change the antioxidative activities, protein and DNA damage [8]. Biological effects of radiation may be primarily due to its thermal effect by an increase in temperature [9]. Though, its non-thermal effects have also been studied [10]. Among the scavenging mechanisms in the cell are the enzymes [8]. Superoxide dismutase (SOD), the first line of defense against ROS, catalyzes the dismutation of the superoxide anion into hydrogen peroxide which can then be transformed into H2O and O2 by catalase [11]. Nitric oxide (NO•) is a small uncharged molecule having a half-life of 2-30 seconds with unpaired electron make it highly reactive and ideal messenger molecule [12]. Three forms of nitric oxide synthase (NOS) catalyze the formation of nitric oxide and citrulline from arginine [13]. It is an important biological molecule that is responsible for cell signaling and host defense against oxidant produced in vivo, but the excess of (NO•) can be toxic [14]. In this connection, peroxynitrite (ONOO -) is unstable molecule and produced in vivo by the reaction of superoxide anion with nitric oxide and this production is regulated mainly by nitric oxide synthase (NOS) and superoxide dimutase (SOD). One of major mechanism of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 966
ISSN 2229-5518
peroxynitrite toxicity is production of hydroxyl (O•H) and carbonate (CO3 • -) radicals. Many harmful roles can be played by peroxynitrite, such as DNA damage, leading to mutagenesis and carcinogenesis. In addition, lipid peroxidation in membranes, causing membrane permeability and fluidity changes. Finally, direct oxidation of transition metal in the active center of the enzymes and nitration of tyrosine residues in different proteins that present in the site [15, 16].
The study was performed on two groups of volunteers were exposed to electromagnetic radiation, the first group was individuals who reside nearby mobile phone base- station antennas (within 100 m around) at least for three years, while the second group was individuals who using commercially available mobile phone heavily (1 5 hours daily not less than one year).These two groups were compared with control group for volunteers who reside faraway from base-station and had using of mobile phone for less or equal to10 minutes. All volunteers were inhabitant at Baghdad city and aged 25±5 years. In addition, they were healthy; nonsmokers adult male and they were of the same socioeconomic status. All chemicals were obtained from BDH and FLUKA companies. Venous blood samples were collected from volunteers into heparinized tubes which were then centrifuged at 3000 g for
10 minutes, plasma and buffy coat were aspirated carefully by Pasture pipette and red blood cells (RBC) were washed twice with equal volume of normal saline solution (0.9% NaCl) and centrifuged at 3000g for 10 minutes, then stored at -20 °C until analyses were carried out. A volume of 1 mL of sediment RBCs was added to four volumes of the cold deionized water to prepare the RBCs lysate. Then ethanol/chloroform extract was prepared by adding 1 volume of hemolysate to 3 volume of ice-cold deionized water followed by 1 volume of cold ethanol, then 0.6 volume of cold chloroform in ice bath. The solution should be mixed after each addition and finally shaken well for about five minutes. Then the tubes were centrifuged at 3000 g for 10 minutes. The clear top layer was used to determine the following parameters in RBC: the protein concentration, superoxide dismutase (SOD) activity, nitric oxide concentration and peroxynitrite concentration.
The protein concentration in RBCs extract of all samples was determined by simple Lowry's method [17].
Superoxide dismutase (SOD) activity was determined according to riboflavin/NBT method [18].
Nitric oxide concentration in RBCs extract was determined according to Jose et al [19]. Nitrite levels in extract RBCs were measured by Griess reaction. Reduction of nitrate to nitrite was accomplished by catalytic reaction using cadmium. Absorbance of this complex was measured at 545 nm. A standard curve was established with a set of serial dilutions of sodium nitrite. Linear regression was made by using the peak area from the nitrite standard. The resulting equation was then used to calculate the unknown sample concentrations and the results were expressed as micromoles per liter.
The peroxynitrite mediated nitration of phenol was measured spectrophotometrically according to the method of Vanuffelen et al [20].
The results were expressed as a mean value ±SD. Comparison of mean values among experimental groups was performed using Student’s t-test. P < 0.05 was considered statistically significant.
There is no enough information available in the literatures about the change in the amount of protein response to mobile phone radiation in humans [21]. Therefore protein concentration in RBCs lysate of the studied samples was measured after hemoglobin removal by adding ethanol and chloroform [18].
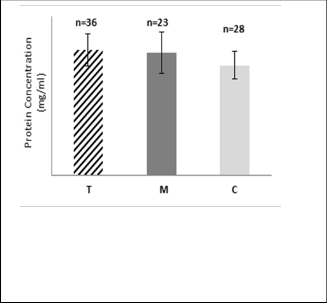
Fig.1: Mean value of protein concentration (mg/ml) in RBC of group T*, M** and C***. (*T: Volunteers who reside nearby mobile phone base- station antennas. ** M: Volunteers who used commercially available mobile phone heavily. *** C: Control group for individuals who reside faraway from base- station antennas and had using mobile phone for less, or equal to 10 minutes daily).
The results in Fig.1 reveal presence of a very highly significant increase in protein concentration in RBC of group T
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 967
ISSN 2229-5518
compared with that of group C volunteers (P < 0.05) also this increase was highly significant in the lysate of group M compared with that of group C stress proteins which are induced by a variety of potentially harmful extracellular stimuli. The role of these proteins is to maintain the structure and function of cellular proteins. The earlier studies showed that ELF induces heat shock protein [26,27,28,29]. Karinen et al. reported that protein expression in human skin might be affected by the exposure to RF-EMF from mobile phone [21]. The results of the presented study disagree with Kula et al who found that significant decreases in the levels of total protein in serum of steelworkers exposed to electromagnetic field [30], and also disagree with El-Abiad and Marzook who showed a significant reduction in total serum protein in old rats exposed to radiation from mobile base station [31]. In addition, disagree with Hassan who observed significant decrease in total protein concentration in rats exposed to electromagnetic field [32]. While Aghazadeh et al found that protein concentration significant increased when rats were radiated with gamma dose [33].
Superoxide dismutase (SOD) activity in RBC was measured in all samples for each group according to riboflavin/NBT method [18]. The amount of superoxide dismutase giving 50% inhibition (SOD50) of an O2 ●-dependent reaction is defined as one unit of SOD activity [34].
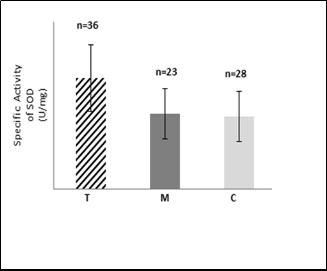
Fig.2: Mean values of specific activity (U/mg tot. protein) of SOD in
RBC of group T, M and C.
The results as shown in Fig.2 reveal high significant increase in SOD specific activity (P < 0.05) in RBCs samples of group T, but the differences were non-significant (P > 0.05) in RBCs samples of group M, in comparison to that of the group C. It is well known that the erythrocytes are particularly vulnerable to oxidative damage due to continuous exposure to high oxygen tension as well as the presence of large amounts of iron, a potent catalyst for oxygen free polyunsaturated fatty acids which are the major targets for peroxidation [35]. The
present results agree with that of study conducted by Kula et al, which pointed that activity of SOD increased of rats exposed to magnetic fields [30]. Also agree with some studies which reported a significant increase in SOD activity in animals exposed to mobile phone radiation [36, 37, 38]. On the other hand, the present results were in contrast with results of study which reported that activity of SOD in human erythrocytes was significantly decreased after exposure to radiofrequency fields of the mobile phone [39]. In vitro, microwaves produced by mobile phones significantly depleted SOD activity in human blood platelets after exposure to radiation[40], but other author indicated that RF radiation did not alter SOD in J774.16 cells [41]. Other researchers found SOD activity decreased significantly in the animal tissues or cells exposed to mobile phone radiation or radiation with same frequency [42, 43, 44, 45, 46]. One important point should be considered when someone look at the disagreement in the results among different reports is the differences in technical features of used devices in the experiences. The normal erythrocyte is resistant to oxidative damage, because it is rich in SOD, CAT, reduced glutathione, glutathione peroxidase, glutathione S- transferase, and vitamin E [47, 48]. Superoxide dismutase metabolizes free radicals and dismutates superoxide anion (O2 ●-) to (H2O2), and protects the cell against O2●-ˉ mediated lipid peroxidation [47]. Since there is an enhancement of free radical activity that causes endothelial damage, the body raises the level of its antioxidants in order to combat such oxidative stress or oxidative damage [49]. Dismutation of increased superoxide radicals, in particular, can be achieved by high SOD activity [50].The increased activity of SOD result in overproduction of H2 O2 which is the product of dismutation reaction, and it has been reported that H2 O2 suppresses SOD activity and superoxide radicals O2 ●- inhibit catalase activity which remove H2 O2 [51]. Also the reactive oxygen species (ROS) production can induce the expression of several genes of antioxidant enzymes such as SOD; catalase [52].The frequencies of oscillation of groups of atoms in the active center of an enzyme are located in the range of 10-100 GHz. The approximate resonant frequencies in Hz have been determined experimentally for a few structures in living cells [53]. Mobile phone radiation induction of free radical formation in some tissues has been reported [54, 36] but the direct biological effects of exposure to 900 MHz RF radiation have not been studied extensively. Only biochemical reactions that involve more than one unpaired electron will be affected by a magnetic field [55]. Though, it is difficult to understand the implicit mechanism for radiation related oxidative stress [56, 57].The results in the present study suggest that ROS were generated under the experimental conditions employed. The observed increased activity may be caused by two factors: (1) increased expression of SOD gene and/or (2) changes in physical properties of SOD. Some of post-translational modifications may be change SOD activity [58]. One study has demonstrated that alkylation of CuZn SOD enhances its structural stability [59] this may lead to dimensioned rate of degradation of enzyme; thus, resulting in a higher
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 968
ISSN 2229-5518
concentration of the enzyme being present in the cells. Another possibility is that the greater stability of the CuZnSOD enzyme might lead to an elevated activity of the enzyme [58]. Modification of the catalytic activity of an enzyme by allosteric effectors is well established in enzymology [60].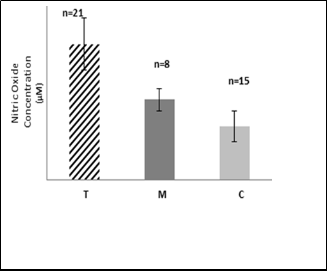
radiation is known to increase the activity of the transcription factor NF-jB [64]. It is well- established that NF-jB activity produces increased synthesis of the inducible nitric oxide synthase (iNOS) which increases, in turn; nitric oxide levels [65, 66]. The toxic effect of nitric oxide can be divided into two categories; direct effect resulting from binding to Fe containing proteins and it is well known hemoglobin forms about 95% of the intracellular protein of the RBCs, the ferrous iron of hemoglobin is susceptible to oxidation by different oxidizing agents, forming methemoglobin, which cannot transport oxygen [67]. Since the main function of red blood cells is to transfer oxygen to tissues, it is exposed to high oxidative stress levels [68] and this will be the case when one live nearby the mobile base station and, or use the mobile heavily every day.
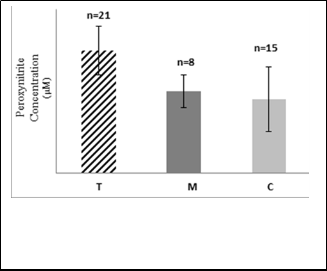
Fig.3: Mean value of nitric oxide concentration (µM) in RBC of group
T, M and C.
In the current study, nitric oxide concentration in RBCs samples of the studied groups was determined by the method based on the reduction of nitrate to nitrite by cadmium and the nitrite produced being determined by Griess reaction [19].
The present results in the Fig.3 show presence of high
significant increase in nitric oxide concentration in RBC of
group T and group M compared with that of C group
volunteers (P < 0.05 in both). Only few data about NO• levels in animal beings including human after exposure to mobile phone have been published. The current results agree with results of some earlier studies which measured NO• concentration using rats exposed to different frequencies of electromagnetic radiation and different conditions [38,54,
61,62]. On the other hand, Irmak et al observed decreased NO• levels in rats exposed to 900 MHz mobile phones radiation [36]. The increased NO• levels in the exposure groups may be related to the stress conditions formed by the radiation in order to scavenge O2•and may act as a defense mechanism presumably related to tissue damage; mobile phones might induce vasodilatation and increase the production of NO• [56]. Treatment of human endothelial cells with UV radiation resulted in an increase of both NO• and ONOO- release. The amount of NO• released by UV-irradiated endothelial cells in the presence of SOD was much higher than in its absence, suggesting the neutralization of NO• by O2• with subsequent formation of ONOO- . The reaction between NO• and O2 • released by endothelial cells is limited by the rate of diffusion of the two radicals. Control of the O2 • and NO• reaction may be the function of the extracellular SOD enzymes which bind to endothelial cell surfaces [63]. In this connection, ionizing
Fig.4: Mean value of peroxynitrite concentration (µM) in RBC of group
T, M and C.
The present results in the Fig.4 that highly significant increase in peroxynitrite concentration in RBC of group T and group M compared with that of C group (P < 0.05 in both). There are no findings in the literatures illustrate the effect of mobile phone radiation on the peroxynitrite concentration. It is worth mentioning that NO• rapidly reacts with superoxide radicals and generates cytotoxic peroxynitrite. Many of authors reported that NO• reacts with endogenously generated superoxide radicals and the resultant ONOO- [65,
69]. Peroxynitrite, although not a free radical, it is a strong oxidizing agent that is stable and directly toxic. At physiological pH, It can diffuse through the cell and lipid membranes to interact with a wide range of targets, including protein methionine, oxidation of sulfhydryl groups of proteins [70], lipids, nitrate free tyrosine and tyrosine residues in proteins to form 3- nitrotyrosine (3-NT), which is a stable product that provides ONOO- mediated tissue damage [71]. It also breaks down to form additional RNS, including the free radical nitrogen dioxide (NO•), an effective initiator of lipid peroxidation and single stranded breaks and modification of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 969
ISSN 2229-5518
bases in DNA, leading to mutagenesis and carcinogenesis [69,
72, 15].
Out of the present study’s results, it can be concluded that living near the mobile base station, and using the mobile heavily have bad effects on human health due to increase the concentration of peroxynitrite. This compound can damage the cell by interfering with proper functions of the different biomolecules, which can in turn cause many diseases like cancer.
We want to gratefully acknowledge the support and facilities of the Department of Chemistry/ College of Science/ University of Baghdad.
[1] D. Panagopoulos. In: Analyzing the health impacts of modern telecommunications microwaves. Berhardt L. Advanced in Medicine and Biology. USA, Nova Science Publishers; 2011, 17:1-55.
[2] D. Panagopoulos, E. Chavdoula, L. Margaritis. Bioeffects of mobile telephony radiation in relation to its intensity or distance from the antennas. Int J Radiat Biol. 2010;86(5):345-357.
[3] G. Hyland. Physics and biology of mobile telephony. The Lancet,
2000;356:1833-1836.
[4] D. Harman. Ageing: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298-300.
[5] N. Desai, R. Sharma, K. Makker, E. Sabanegh, A. Agrawal. Physiologic
and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92(5):1626-1631.
[6] J. Bogdanska, P. Korneti, B. Todorova. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in republic of Macedonia. Bratisl Lek Listy.2003;104(3):108-114.
[7] R. Srikrishna, D. Suresh. Biochemical study of antioxidant profile in acute ischemic stroke. BJMP.2009;2(1):35-37.
[8] J. Jajte, J. Grzegorczyk, M. Zmyslony, E. Rajkowska. Effect of 7 mT static magnetic field and iron ions on rat lymphocytes: apoptosis, necrosis and free radical processes. Bioelectrochem. 2002; 57(2):107-111.
[9] N. Desai, K. Kesari, A. Agarwal. Pathophysiology of cell phone
radiation: oxidative stress and carcinogenesis with focus on male reproductive system. Rep Biol Endo. 2009; 7:114.
[10] J. Friedman, S. Kraus, Y. Hauptman, Y. Schiff, R. Seger. Mechanism of short-term ERK activation by electro-magnetic fields at mobile phone frequencies. Biochem J. 2007;405(3):559-568.
[11] J.Van Raamsdonk, S. Hekimi. Superoxide dismutase is dispensable for normal animal lifespan. PNAS. 2012;109(5):5785-5790.
[12] J. Charless, L. Jay, H. Solomon. Nitric Oxide: A Physiologic
Messenger. Annals of Internal med. February, 1994; 120(3): 227-237.
[13] H. Horton, L. Moran, R. Ochs, D. Rawn, K.Scrimgeour. Principle of
Biochemistry. 3th ed. USA, Prentice Hall; 2002.
[14] R. Cannon. Nitric oxide in cardiovascular disease. Clin Chem.
1998;44(8):1809-1819.
[15] P.Pacher, J.Beckman, L. Liaudet. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424.
[16] F.Yamakura, H.Taka, T.Fujimura, K.Murayama. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrosine. The J Biol Chem.
1998;273:14085-14089.
[17] O. Lowry, N. Roserbongh, Farr N. Protein measurement with the
Folin phenol reagent. J Biol Chem. 1951;193:265-275.
[18] C. Winterboum, R. Hawkins, M. Brian, R. Carrell. The estimation of red cell SOD activity. J Lab Clin Med.1975; 85(2):337-341.
[19] A. Jose, G. Cristina, A. Joaquin. Determination of nitric oxide. Clin
Chem. 1998;44(3):679-681.
[20] B. Vanuffelen, J. Vandevzee, B. koster, k. Stevenin, J. Elferink. Assay of peroxynitrite. Biochem J. 1998;330:719-722.
[21] A. Karinen, S. Heinavaara, R. Nylund, D. Leszczynski. Mobile phone radiation might alter protein expression in human skin. BMC Genomics. 2008;9:77-86.
[22] E. Gaafar, M. Hanafy, E.Tohamy, M. Ibrahim. The effect of electromagnetic field on protein molecular structure of E. coli and its pathgenesis. Romanian L Biophys. 2008;18(2):145-169.
[23] W. Edgar. Saliva: its secretion, composition and functions. Brit Den J.
1992; 172:305-312.
[24] F. Zilva, P. Mayne. Clinical Chemistry in Diagnosis and Treatment. 6th ed. Arnold London; 2002.
[25] L. Kaplen, A. Pesce, S. Kazmierczack. Clinical
Chemistry:Theory,Analysis,Correlation.4th ed. Mosby; 2003.
[26] D. Weisbrot, H. Lin, L. Ye, M. Blank, R. Goodman. Effect of mobile phone radiation on reproduction and development in Drosophila melanogaster. J of Cell Biochem. 2003; 89:48-55.
[27] R. Goodman , D. Weisbrot , A. Uluc , A. Henderson . Transcription in Drosophila melanogaster salivary gland cells is altered following exposure to low-frequency electromagnetic fields: analysis of chromosome 3R. Bioelectromagn. 1992;13(2):111-118.
[28] K. Fritze, C. Wiessner, N. Kuster, et al. Effect of global system for
mobile communication microwave exposure on the genomic response of the rat brain. Neuroscience. 1997;81:627-639.
[29] D. de Pomerai, C. Daniells , H. David. et al. Non- thermal heat shock response to microwaves. Nature.2000;405(6785):417-418.
[30] B. Kula, A. Sobczak, R. Bochenek, D. Piskorska. Effect of Electromagnetic Field on Serum Biochemical Parameters in Steelworkers. J Occup Health.1999;41:177-180.
[31] NM. El-Abiad, EA. Marzook. Effect of environmental microwave radiation exposure emitted from cellular phone base station on some biochemical parameters in rat. Sci Med J. 2005;17(1):69-78.
[32] B. Hassan. Sub chronical effects of electromagnetic field exposure of adult female rats on some hormonal, biochemical and hematological parameters. Diyala Agr Sci J. 2011;3(1):47-53.
[33] S. Aghazadeh, M. Azarnia, A.Shirazi, S. Mahdavi, B. Zanjii. Protective
effect of melatonin on spinal cord damage after gamma irradiation.
AATEX 14, Special Issue. 2007;535-538
[34] H. Misra, I. Fridovich. Inhibition of superoxide dismutases by azide.
Arch Biochem Biophys. 1978;189(2):317-322.
[35] M. Scott, B. Lubin, L. Zuo, F. Kuypers. Erythrocyte defense against hydrogen peroxide, preeminent importance of catalase. J Lab Clin Med.1991;118(1):7-16.
[36] M. Irmak, E. Fadillioglu, M. Gulec, H. Erdogan, M. Yagmurca, O.
Akyol. Electromagnetic radiation from a cellular telephone on the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 970
ISSN 2229-5518
oxidant and antioxidant levels in rabbits. Cell Biochem Funct.
2002;20(4):279-283.
[37] A. Yurekli, M. Ozkan, T. Kalkan, et al. GSM base station electromagnetic radiation and oxidative stress in rats. Electro Biol And Med.2006;25(3):177-188.
[38] F. Ozguner, Y. Bardak, S. Comlekci. Alteration in ipid peroxidation, cytochrome P450 glutathione and its metabolizing enzymes upon monosodium glutamate administration in hepatic tissue of adult male mice. Molecul and Cell Biochem.2006;277:73-80.
[39] Y. Moustafa, R. Moustafa, A. Belacy, S. Abou-EL-Ela. Effect of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. J Pharm Biomed Anal. 2001;26(4):605-608.
[40] D. Stopczyk, W. Gnitecki, A. Buczynski, L. Markuszewski, J.
Buczynski. Effect of electromagnetic field produced by mobile phones on the activity of SOD-1 and level of malonyldialdehyde -in vitro study. Med Pr.2002;53(4):311-314.
[41] H. Graham, S. Doglas, Julia S. Evaluation of parameters of stress after in vitro exposure to FMCW and CDMA-modulated radiofrequency radiation field. Radiation Research, 2004;162(5):497-504.
[42] A. Ayata, H. Mollaoglu, H. Yilmaz. Oxidative mediated skin damage in an experimental mobile phone model can be prevented by melatonin. J Dermatol. 2004;31(11):878-883.
[43] F. Oktem, F. Ozguner, H. Mollaoglu, A. Koyu, E. Uz. Oxidative
damage in the kidney induced by 900 MHz-emitted mobile phone: Protection by melatonin. Arch Med Res. 2005;36(4):350-355.
[44] K. Kesari, J. Behari. Whole body 900 MHz radiation exposure effect on enzyme activity in male wistar rats Bioelect. 2007;19:57-66.
[45] M. Balci, E. Devrim, L. Durak. Effects of mobile phones on oxidant/antioxidant balance in cornea and lens of rats. Eye Res.
2007;32(1):21-25.
[46] S. Awad, N. Hassan. Health risks of electromagnetic radiation from mobile phone on brain of rats. J of App Sci Res. 2008;4(12):1994-2000.
[47] JM. Mates, C. Perez-Gomez, I. Nunez de Castro. Antioxidant enzymes and human diseases. Clin Biochem.1999;32(8):595-603.
[48] AH. Abou Ghalia, IM. Fouad. Glutathion and its metabolizing
enzymes in patients with different benign and malignant diseases.
Clin Biochem. 2000;33(8):657-662.
[49] D. Scully, S. Langley-Evan. Salivary antioxidants and periodontal disease status. Proce Nutr Soci. 2002;61(1):137-143.
[50] J. Lukac, M. Mravak-Stipetic, M. Knezevic, et al. Phagocytic functions of salivary neutrophils in oral mucosa membrane disease. J Oral Pathol Med. 2003;32(5):271-274.
[51] P. Sient, P. Garber. Inactivation of the human CuZn Superoxide dismutase during exposure to O 2 .- and H 2 O2 . Arch of Biochem and Biophys.1981;212(2):414-416.
[52] T. Dalton, H. Shertzer, A. Puga. Regulation of gene expression by reactive oxygen. Annu Rev pharmacol Toxicol. 1999;39:67-101.
[53] V. Illarionov. Medical Informational-Wave Technology. Moscow: VTs
MK. Zashchita;1998.
[54] A. Ilhan, A. Gurel, F. Armutcu, et al. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Acta.
2004;340(1-2):153-162.
[55] N. Buyukuslu, O. Celic, C. Atak. The effect of magnetic field on the activity of superoxide dismutase. J of Cell and Mol Biol. 2006;5:57-62.
[56] S. Dasdage, H. Bilgin, M. Akdag, H. Celik. F. Aksen. Effect of long term mobile phone exposure on oxidative-antioxidative processes
and nitric oxide in rats. Biotechnol & Biotechnol Equip. 2008;22(4):992-
997.
[57] A. Achudume, B. Onibere, F. Aina. Bioeffect of electromagnetic base station on glutathione reductase, lipid peroxidation and total cholesterol in different tissues of Wistar rats. Biol and Med.
2009;1(3):33-38.
[58] S. Manhas. Levels of CuZnSOD in erythrocytes of Alzheimer's disease patients and normative aging subjects. M.Sc.Thesis. Simon Fraser University, Canada. 1995.
[59] J. Jabusch, D. Farb, D. Kerschensteiner, H. Deutsch. Some sulfhydryl properties and primary structure of human erythrocyte superoxide dismutase. Biochem.1980;19(11):2310-6.
[60] T. Devlin. Text Book of Biochemistry with Clinical Correlations. 6th ed.
USA. Wiley-Liss; 2006.
[61] P. Paredi, S. Kharitonov, T. Hanazawa, P. Barnes. Local vasodilator response to mobile phones. Laryngoscope, 2001;111(1):159-162.
[62] M. Yariktas, F. Doner, F. Ozguner, O. Gokalp, H. Dogru, N. Delibas.
Nitric oxide level in the nasal and sinus mucosa after exposure to electromagnetic field.Otolaryngol Head Neck Surg. 2005;132(5):713-716.
[63] G. Deliconstantinos, V. Villiotou, J. Stavrides. Nitric oxide and peroxynitrite released by ultraviolet B-irradiated human endothelial cells are possibly involved in skin erythema and inflammation. Exper Physiol. 1996;81(6):1021-1033.
[64] Y. Ibuki, S. Mizuno, R. Goto. γ-Irradiation-induced DNA damage
enhances NO production via NF-κB activation in RAW264.7
cells. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2003;1593(2-
3):159-167.
[65] M. Pall. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypoth. 2000;54(1):115-125.
[66] H. Kleinert, P. Schwarz, U. Forstermann. Regulation of the expression of the inducible nitric oxide synthase. Biol Chem. 2003;384(10-
11):1343-1364.
[67] R. Murray, D. Granner, P. Mayes, V. Rodwell. Harper's Illusrated
Biochemistry. 26th ed. USA: The McGraw-Hill Companies; 2003.
[68] Z. Joswiak, B. Jasnowska. Changes in oxygen-metabolizing enzymes and lipid peroxidation in human erythrocytes as a function of age of donor. Mech Aging Dev.1985;32(1):77-83.
[69] K. Nagata, H. Yu, M. Nishikawa, et al. Helicobacter pylori generate superoxide radicals and modulates nitric oxide metabolism. J of Biol Chem. 1998; 273 (23):14071-14073.
[70] I. Takeda, Y. Kizu, O. Yoshitaka, I. Saito, G. Yamane. Possible role of nitric oxide in radiation-induced salivary gland dysfunction. Radiat Res. 2003;159:465–470.
[71] A. Gow, C. Farkouh, D. Munson, M. Posencheg, H. Ischiropoulos.
Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):262–268.
[72] A. Marks. Lieberman M. Basic medical biochemistry: A clinical approach.
2nd ed. USA: Lippincott Walliams & Wilkins, aWolters Kluer
Company; 2005.
IJSER © 2014 http://www.ijser.org