International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1070
ISSN 2229-5518
Effect of Electroless Cu Coating on the Mechanical properties of Al6061/SiC/Gr based
Hybrid Composite
Mohamed Zakaulla, A.R.Anwar khan, Sanaulla P.F, P.G.Mukunda.
Abstract— — Al6061 alloy reinforced with both Cu coated SiC particles and Cu coated Graphite powder are prepared by stir casting technique. SiC particles are used to increase the hardness of composite while Graphite powder acts as a solid lubricant. SiC particles and Graphite powder are coated with a copper layer through electroless deposition method. The effect of PdCl2 concentration and time of stirring of the activated particles in electroless solution are reported. XRD analysis indicated the presence of copper on both the surfaces of SiC and Gr. It was found that density, hardness and tensile strength of the hybrid composites increased when coated reinforcements were used in compare to uncoated reinforcements. Dimple formation, voids, fractured particles and areas of brittle fracture are observed on the fracture surfaces of the tensile specimens. Copper coating on the reinforcements improved the ductility due to improved wettability.
Index Terms— Cu coated SiC, Cu coated Gr, Dimple formation, Electroless, hybrid composite, stir casting, wettability.
—————————— ——————————
1 INTRODUCTION
Hybrid composites, particularly Al/SiC/Gr have attracted greater importance in recent years to improve or modify prop- erties such as strength, stiffness, wear resistance and thermal expansion characteristics of monolithic aluminium alloys [1-3]. Al/SiC/Gr hybrid composite are used in automobile industry such as manufacture of pistons, cylinder liners, brake disks, bushings and brake rotors. An important consideration in the manufacture of metal matrix composites is to prevent the chemical reaction between SiC and Gr with Al matrix which leads to the formation of brittle Al4 C3 phase.
4Al + 3SiC = Al4 C3 + 3Si.
This Hygroscopic carbide results in the degradation of me-
chanical properties even if is present in small quantities [4-6].
Graphite which acts like a solid lubricant improves not only
antifriction properties but also wear and machining properties
[7, 8]. Rohatgi et al. have concluded that friction coefficient of
Al-10%SiC-6%Gr is very low due to combined addition of SiC
which increases bulk mechanical properties and formation of
graphite film [9].Basavarajappa et al. on Al-15%SiC-3%Gr
composites have reported that wear rate in graphite compo-
sites is less than that of graphite free composites and indicated
degree of subsurface deformation [10]. When Graphite is in-
serted in to Al matrix, they dissolve in Al matrix and tend to
float during solidification because of inherent difference in
density between Al and graphite and also results in non uni-
form distribution and poor wettability. A common solution to
the above problems is to coat the SiC & Gr with copper [11-12]
or nickel [2] to increase its wettability, density and its uniform
distribution in the matrix. Another alternative to increase the
wettability is to heat the Graphite at 400 ○C in order to elimi-
nate gaseous elements, but this method is less efficient then
coating the graphite with thin layers. Copper coating using
electroless method is a versatile process to coat metallic copper
on ceramic surfaces without consuming any external electric
power. Successful coating of particles such as mica [13], iron [14] and fly ash [15] by electroless method has been reported earlier in the literature. In the present investigation in order to improve wettability and uniform distribution of SiC and Gr, Electroless copper coating technique was used. Hybrid com- posites were manufactured with uncoated and Cu coated rein- forcements using vortex technique. Microstructure and me- chanical properties of manufactured composites were evaluat- ed and it was found that mechanical properties improved when coated reinforcements were used instead of uncoated reinforcements.
2. EXPERIMENTAL PROCEDURE
2.1. Electroless copper coating on silicon carbide particles and Graphite
TABLE 1. Steps in Electroless copper coating.
————————————————
Mohamed Zakaulla, Mechanical, H.K.B.K.C.E, Bangalore, Karnataka, India.
Dr.A.R. Anwar Khan, mechanical, Ghousia college of Engineering, Banga-
lore, Karnataka, India.
Dr.Sanaulla P.F, Chemistry, H.K.B.K.C.E, Bangalore, Karnataka, India.
Dr.P.G.Mukunda, Mechanical, Nitte institute of technology, Bangalore,
Karnataka, India.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1071
ISSN 2229-5518
TABLE 2. Electroless copper solution.
Chemical | Concentration |
Copper sulphate (CuSo4 .5H2 O) | 20g/l |
Sodium hydroxide | 20g/l |
Potassium sodium tar- trate | 100g/l |
Na2 EDTA | 20g/l |
Formaldehyde. | 20ml/l |
The Electroless copper coating on silicon carbide particles and Graphite powder relies on sequence of cleaning, sensitising, activating and plating. The procedure and chemicals used are indicated in Tables 1and 2 respectively.
2.2 Scheme of reactions for Electroless deposition of cop- per.
The overall reaction for electroless deposition of copper, with formaldehyde (HCHO) as the reducing agent, is
Cu2+ + 2HCHO + 4OH¯ → Cu + 2HCOO¯ + 2H2 O + H2
…….(1)
Where HCOO¯ is formic acid is the oxidation product of the reducing agent.
The fundamental aspects of this reaction are presented in Equation (2) and (3) which interpretate electroless deposition of copper on SiC and graphite. The overall reaction, given in equation (1), can be explained in to a simple reduction reac- tion, the cathodic partial reaction and oxidation reaction, the anodic partial reaction.
Reduction of copper ion.
Catalytically activated surface (PdCl2 + SnCl2 )
Cu2+ (ion) + 2e 
Cu (lattice) ……… (2)
Oxidation of reducing agent HCHO.
der was added to remove slag. The mechanical stirrer was in- serted into it and rotated at a desired rpm to create necessary vortex. Preheated uncoated or coated reinforcements were added into the vortex. Melt was stirred for at least 10 minute. Stirring was stopped and stirrer was taken out of crucible. The crucible was taken out of the furnace and melt was poured into permanent moulds.
3. RESULTS AND DISCUSSION:
3.1 Effect of concentration of Palladium Chloride (PdCl2 )
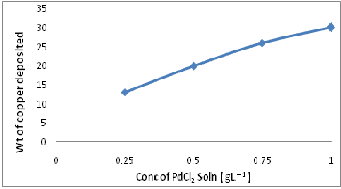
Fig 1 shows the effect of PdCl2 concentration on the weight percent of copper deposited on the SiC particle surface. It is observed that the weight percent of copper deposited changes from 15 to 30 with a change in PdCl2 concentration from 0.25 to 1 g l -1 of PdCl2.
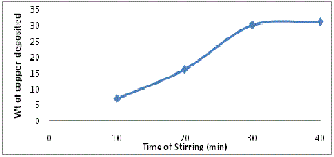
Fig.1 weight percent copper deposited as a function of concen- tration of PdCl2 Solution.
3.2 Effect of stirring time in electroless bath
HCHO +
………… (3)
→2OH¯ H COO¯ + 2H 2 O + H2
Fig.2 Weight percent copper deposited as a function of time of stirring in electroless bath
2.3 Processing:
The Experimental setup used for preparing the composite con- sists of Electrical resistance furnace and a stirrer. About 3kg of Al alloy was melted in a crucible. The metal was superheated to 8000 C and then degassing was carried out using degassing tablet ( exo chloroethylene) to remove oxygen and scum pow-
Basically coating reaction starts when SiC particles are activat- ed by PdCl2 and are dispersed in the coating solution. Palladi- um metal at the surface of SiC particles acts as a catalyst. In the electroless bath as the stirring time of SiC particles increas- es the percent of copper deposited also increases up to a stir- ring time of about 30 min as shown in fig 2. The colour of electroless solution changes progressively as reaction pro-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1072
ISSN 2229-5518

gresses due to depletion of copper and for stirring time of 30 mins, 29 to 30 wt% of copper is deposited. There is no increase in copper deposited on SiC particles after a stirring time of 30 mins because there is precipitation of copper in the solution instead of further deposition of the copper on SiC surface.

3.3 SEM analysis
Fig.6 SEM image of Cu coated Graphite powder
Fig 5 & 6 shows the surface of uncoated and Cu coated
Graphite powder.

Fig.3 SEM image of uncoated SiC particles 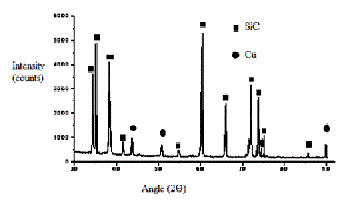
3.4 XRD analysis
Fig 7. XRD of copper-coated silicon carbide particles
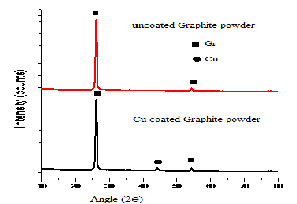
Fig.4 SEM image of Cu - coated SiC particles.
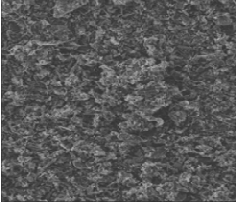
Fig 3 & 4 shows the surface of uncoated and Cu coated angu- lar silicon carbide particles.
Fig 8. XRD of copper-coated Graphite powder
Fig.5 SEM image of Uncoated Graphite powder
From fig 7 & 8, an X-ray diffraction study clearly indicates the presence of copper on SiC and Gr. Hence it can be concluded that after Electroless coating process, Cu has been successfully deposited on SiC particle surface and graphite powder.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1073
ISSN 2229-5518

3.5 Microstructure
The microstructure of uncoated SiC and Gr based Al composites examined under optical microscope are shown in fig 9a-d. Micro- structure shows SiC particles are distributed randomly throughout the matrix in compare to graphite.
Fig 10a – c shows the microstructure of both Cu coated SiC and Gr based Al composites. It is observed that there is uniform dis- tribution of Cu coated reinforcements within the matrix since Cu coating using electroless technique enhances the interfacial bond- ing between the coated reinforcements and the matrix.

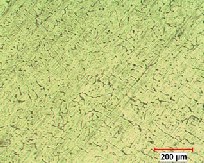 (d) Al6061-8% Sic-1% Gr
(d) Al6061-8% Sic-1% Gr
Fig 9 (a) Al6061
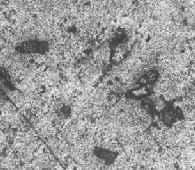
 Fig 10 (a) Al6061 + Cu-2% Sic + Cu-1% Gr
Fig 10 (a) Al6061 + Cu-2% Sic + Cu-1% Gr
(b) Al6061-2% Sic-1% Gr
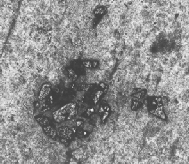
 (b) Al6061 + Cu-4% Sic + Cu-1% Gr
(b) Al6061 + Cu-4% Sic + Cu-1% Gr
(c) Al6061-4% Sic-1% Gr
c) Al6061 + Cu-8% Sic + Cu-1% Gr
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1074
ISSN 2229-5518
3.6 Density
Generally rule of mixtures was used to calculate the theoreti- cal densities. Experimental density of manufactured compo- sites was found by Archimedes principle. The composite samples were first weighed in air and then tied with string and weighed while hanging in water. The density was deter- mined using the following formula:
ρc = (ma x ρw )/ (ma - mw)
Where,
ρc = Density of specimen (Kg/ m3)
ρw = Density of water (Kg/m3)
ma = Weight of sample in air (kg)
mw = Weight of sample in water (kg).
The experimental density was also calculated by measur- ing the weight and volume of the specimens. The volume was determined by measuring the accurate dimensions of the spec- imen. Fig 11and 12 shows the theoretical and experimental densities of manufactured composites having uncoated and Cu coated SiC and Gr. Figure 11 shows linear increase in the experimental densities, the values are lower than that of the theoretical densities (as expected from the rule of mixtures). The increase in density of composites is due to the presence of increasing content of SiC particles which is hard and brittle and leads to the dispersion hardening of matrix. Figure 13 shows the experimental density of manufactured composites having uncoated and Cu coated reinforcements. It is revealed that there is rise in density when Cu coated reinforcements are used in compare to uncoated reinforcements because it forms Al-Cu liquid eutectic and its flow in to porous areas helps in reducing the pores. In addition when SiC and Gr are coated with copper it forms Al2 Cu phase which have high density value in compare to density of pure aluminium. D. Mandal et al. [14 ] in their work on Effect of wt% reinforcement on micro- structure and mechanical properties of Al–2Mg base short steel fiber composites state that density of composites was higher compared to Al–2Mg matrix and also increases with increasing wt% of Cu coated steel fibers.
The porosity values are obtained from the theoretical density values calculated using the rule of mixtures. It is observed from fig.14 that % of porosity increases with increasing wt% of Cu coated reinforcements in Al matrix.
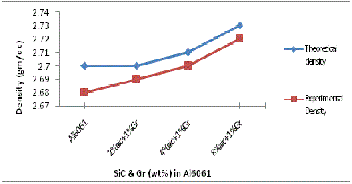
Fig. 11 Theoretical & Experimental Density variation with wt%
of SiC & Gr addition in Al6061/SiC Gr composite
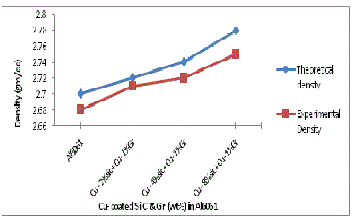
Fig. 12 Theoretical & Experimental Density variation with wt% of Cucoated SiC & Gr addition in Al6061/SiC/ Gr compo- site.
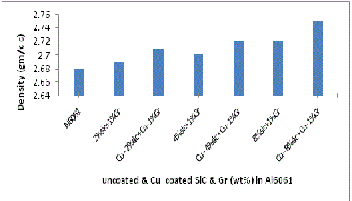
Fig.13.Experimental Density variation with wt% of Uncoated
& Cu- coated SiC & Gr addition in Al6061/ SiC/G composite
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1075
ISSN 2229-5518
3.8 Tensile properties
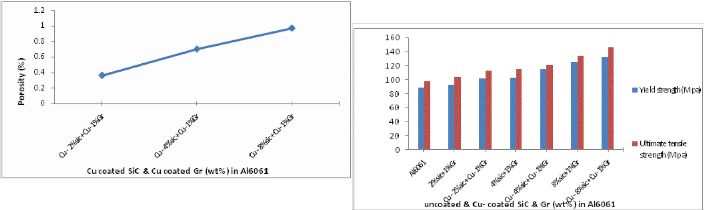
Fig 14. Porosity variation with wt% of Cu- coated SiC & Gr addition in Al6061/SiC /Gr composite.
3.7) Hardness
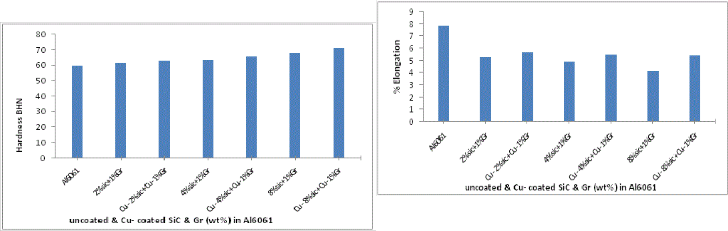
Fig. 15 Variation of hardness of uncoated and Cu coated SiC & Gr addition in Al6061/SiC/Gr composite.
Hardness of manufactured composites with uncoated and Cu coated reinforcements are shown in fig 15. It is observed that as the SiC content is increased the hardness of composite also increases due to the increase in the percentage of the hard and brittle phase of the ceramic body in the alloy. Adding of Graphite does not positively contribute to the hardness of the composite. Maximum hardness was observed in case of Cu -
8% SiC +Cu-1%Gr based Al composite. Diffusion of copper in to the matrix increases the hardness of composite. Copper is one of the few elements that have relatively high solubility in Al and when reinforcements are coated with copper it forms Al2 Cu phase which is mechanically tougher than pure Al ma- trix. In case of copper-coated reinforcements cleaner and im- proved interface bonding leads to a higher microhardness val- ue.
Fig. 16 Variation of ultimate tensile strength for uncoated and
Cu coated SiC & Gr addition in Al6061/SiC /Gr composite
Fig. 17 Variation of % Elongation for uncoated and Cu coat- ed SiC & Gr addition in Al6061/ SiC/Gr composite.
Fig 16 shows the variation of ultimate tensile strength and yield strength for the manufactured composites for both un- coated and Cu coated reinforcements. It can be seen that the UTS and Yield strength of the composites reinforced by coated reinforcements is obviously higher than that by uncoated rein- forcements because copper coating of SiC & Gr can enhance greatly the wettability and forms a good interface bonding between reinforcements and matrix.
Fig 17 shows the variation of % elongation for all the manufac-
tured composites. It can be seen that ductility is enhanced when coated reinforcements are used instead of uncoated rein- forcements in the composite because of improved wettability and also avoiding the formation of degradation products at interfaces.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1076
ISSN 2229-5518
3.9 Fracture surface

Fig 18 (a) Al6061

(b) Al6061 + 2%SiC + 1%Gr


(c) Al6061 + 4%SiC + 1%Gr
Al6061 + Cu-2%SiC + Cu-1%Gr
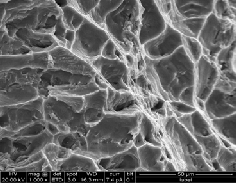
(b) Al6061 + Cu-4%SiC + Cu-1%Gr

(c) Al6061 + Cu-8%SiC + Cu-1%Gr
Fig 19 (a)
(d) Al6061 + 8%SiC + 1%Gr
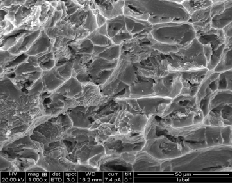
Fracture surface of manufactured composites with varying wt% of uncoated and Cu coated reinforcements are shown in fig 18 a-d & 19 a-c. It is observed that fracture mechanism of Cu coated reinforcements based composite are mainly domi- nated by dimple formation indicating the ductile nature of fracture. In composites containing uncoated reinforcements cracks are initiated at SiC & Gr with Al interface and propa- gate through the interface linking up with other cracks. In Al6061-8%SiC-1%Gr composite shows areas of brittle fracture, voids and fractured particles because of higher level of porosi- ty at the interface associated with increased particle content
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 1077
ISSN 2229-5518
promoting crack initiation and propagation. Baron et al. [15] have reported that steel perform reinforced composites show failure to occur within the interfacial intermetallic phase. Copper coating on reinforcements acts as barrier between SiC/Gr and matrix and also minimizes the formation of reac- tion phases. From the results of tensile test and fractography it is concluded that that intermetalics at Al with SiC & Gr inter- faces makes composite more brittle but copper coating on SiC particles and Gr forms good interfaces which resist the crack propogation.
4. CONCLUSIONS
1) SiC particles and Gr powder were successfully coated with copper using electroless technique in a copper sulphate solu- tion after suitable sensitization and activation treatment.
2) A uniform distribution of Cu coated reinforcements is ob-
tained in the matrices of Al6061 using vortex method.
3) Cu coating improved the wettability of SiC & Gr and en-
hanced the interfacial bonding between the reinforcements and the matrix.
4) Hardness, ultimate tensile strength and ductility of manu-
factured composites increased when reinforcements were coated with copper in comparison to uncoated reinforcements.
5) Fracture mechanism of manufactured composites contain- ing coated reinforcements are dominated by dimple formation indicating the ductile nature of fracture.
5. REFERENCES
1. Ames W. Alpas A.T, wear mechanisms in hybrid composites of graphite – 20 pct SiC in A356 aluminium alloy, Metallurgical and Materials transaction A, volume 26A, January 1995.
2. Ted Guo M.L. Chi, Y.A.Tsao, Tribological behaviour of alumin-
ium/SiC/nickel- coated graphite hybrid composites, Materials
Science and Engineering, A333 (2002), p.134 – 135.
3. A. Urena, J. Rams, M. Campo, M. Sánchez, Effect of reinforce- ment coatings on the dry sliding wear behavior of alumini- um/SiC particles/carbon fibres hybrid composites, wear,
266,(2009), 1128-1136.
4. J.-C. Lee, J.-Y. Byun, C.-S. Oh, H.-K. Seok and H.-I. Lee, Acta Ma- ter. 45 (1997) 5303.
5. A. M. Davidson and D. Regener, Comp. Sci. Techn. 60 (2000)
865.
6. L. M. Tham, M. Gupta and L. Cheng, Acta Mater. 49 (2001)
3243.
7. S. Suresha, B.K.Sridhara, Effect of silicon carbide particulates on wear resistance of graphitic aluminium matrix composites, Ma- terials and Design, 31, (2010), 4470-4477.
8. Soheli Mahdavi, Farshad Akhlaghi,Effect of SiC content on pro- cessing, compaction behavior, and properties of Al6061/SiC/ Gr hybrid composites, journal of material science, 46, (2011), 1502-
1511
9. Rohatgi PK, Guo R Kim JK, Rao S, Stephenson T,wanner T.
Wear and friction of cast Al-SiC-Gr Indianapolis, Indiana: 15-18,
1997, p.205 -211.
10. Basavarajappa S, Chandramohan G, mahadevan Arjun, Tan- gavelu mukundan, influence of sliding speed on dry sliding wear behavior and subsurface deformation on hybrid metal ma- trix composite. Wear 262 (2007),1007- 1012.
11. S.F.Moustafa, S.A.El-Badry, A.M.Sanad, B.kieback, Friction and wear of copper- graphite composites made with Cu-coated and uncoated graphite powders, wear, 253, (2002), 699-710.
12. HU Zhong-liang, CHEN Zhen-hua, XIA Jin-tong, JING Guo- yun, Properties of electric brushes made with Cu-coated graph- ite composites and with copper powders, Transaction of Non Ferrous Metals of China, 17, (2007), 1060-1064.
13. Deonath, P.k.Rohatgi, cast aluminium alloy composites contain- ing Copper-coated ground mica particles, Journal of Material science, 16,(1981), 1599-1606.
14. D. Mandal, B.K. Dutta, S.C. Panigrahi, Effect of wt% reinforce- ment on microstructure and mechanical properties of Al–2Mg base short steel fiber composites, Journal of materials processing technology, 198 ( 2008 ) 195–201.
15. S.Shukla, S.Seal, J.Akesson, R.Oder, R.Cater, Z.Rahman, study
of mechanism of electroless copper coating of fly- ash Ceno- sphere particles, Applied surface science, 181, (2001), 35-50.
16. Baron, R.P., Wert, J.A., Gerard, D.A., Wawner, F.E., 1997. J. Ma- ter.Sci. 32, 6435.
IJSER © 2014 http://www.ijser.org


























