International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 1
ISSN 2229-5518
Dynamic Simulation of Reactor to Produce 1 - Butene by Dimerization of Ethylene
Anurag Choudhary, Avinash Shetty, Satish Oswal
Abstract— The aim of this work was to develop dynamic simulation model for reactor which is heart of 1-Butene pro- duction. The liquid phase catalytic dimerization of ethylene in 1-Butene stands as the most selective and economical route to produce polymerization grade 1-Butene. This paper identifies the components of this homogeneous system and dis- cusses, in fair detail, the factors controlling its selectivity to 1-Butene. Other different themes covered include: structure of the active species, kinetics, mechanistic considerations and the principal reaction parameters. This dimerization is achieved thanks to a titanium compound activated by alkyl aluminum (TEAL). BY-Products are mainly hexenes formed either by reaction of 1-Butene with Ethylene or by trimerization of ethylene, and some small amount of polymer forma- tion. The heat of reaction is removed by heat exchanger provided in pump around loop. The catalyst and TEAL is added in pump around loop in optimum ratio to increase selectivity of 1-Butene. The simulation of such a reactor was performed using the simulation program UNISIM, which does not have provision for non-standard reaction kinetic expressions. Ow- ing to this limitation of the software, the bubble reactor had to be described by dividing reactor in five small continuous stirred types of reactor (CSTR) for optimum performance. The simulation model is very flexible and useful for the simula- tion and operation of this type of reactor.
—————————— ——————————
1-Butene is a basic petrochemical building block of captive requirements, not only it can be converted to polybutene-1 and butylene oxides, but also its largest utilization is as a co-monomer with ethylene for the production of higher strength and higher stress crack resistance polyethylene resins (LLDPE and HDPE).
The liquid phase catalytic dimerization of gaseous ethylene in 1-Butene stands as the most selective and economical route to produce polymerization grade 1- Butene. Titanium based Catalyst LC2253 (Trade Name) and activator Triethyl Aluminum (TEAL) are used for selective 1-Butene production. The march in the catalytic dimerization of ethylene into 1-Butene was pioneered in 1952 by the systematic studies of Ziegler which were originally aimed at producing higher-chain polymers via the growth reaction of the organ aluminum compounds (multiple insertion of ethylene into the Al-C bonds). Ziegler type catalyst based on titanium has a tendency to polymerize ethy-
lene to high molecular weight materials [1].The poly- merization reaction is inhabited in the Alphabutol Cat- alyst by adding a modifying agent to the catalytic for- mula to stabilize the titanium (IV) complex, thus pre- venting the formation of titanium (Ill) complex which is responsible for the polymer formation. The modifier and the titanium compound LC 2253 are mixed under a well-defined ratio and form the active catalyst. The active species are realized by mixing TEAL and LC
2253 catalyst under ethylene pressure.
The aim of this work was to develop dynamic simula-
tion model of 1-Butene reactor operating at a site of RIL for various operational and engineering studies.1- Butene reactor was simulated in ‘UniSim Design’ si- mulation software purchased from Honeywell Interna- tional Inc.
2. PROCESS DESCRIPTION
————————————————
![]() Authors are working in Reliance Simulation Technical Services Depart- ment of Reliance Industries Limited
Authors are working in Reliance Simulation Technical Services Depart- ment of Reliance Industries Limited
Dimerisation reaction occurs in the liquid phase of the
IJSER © 2012
The research paper published by IJSER journal is about Dynamic Simulation of Reactor to Produce 1-Butene by Dimerization of Ethylene 2
ISSN 2229-5518
reactor and in the pump around loop. This liquid phase is formed mainly of two products: Butene and C6+ with the dissolved ethylene feed. The catalyst injected into the pump around loop promotes the reaction. The ethylene feed enters the reactor through a distributor from the bottom to ensure good dispersion of gas bub- ble within the liquid. The gas will dissolve within the liquid medium where the reaction occurs. This dimeri- sation reaction is exothermic in nature, to remove the heat of reaction there are two heat exchangers (in pa- rallel), through them reactor liquid is circulated by us- ing two pumps (in parallel) called as pump around pumps. The effluent from the reactor is withdrawn from the bottom and pumped to the catalyst removal section using two effluent pumps. Fig 1 illustrates process flow of reactor.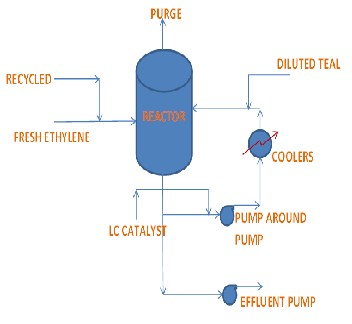
The per-pass conversion in the reactor should be around 80-82 %. Control of this conversion can be achieved by regulation of the temperature and pressure in the reactor. The reactor is operated at the bubble point and its composition is very close to a binary mixture of ethylene and 1-Butene. The pressure in the reactor is the sum of the partial pressure of each com- ponent. Once the temperature is fixed the vapor pres-
sure of each component is imposed.
The temperature of the reactor can be controlled by action on the cooling water flow to the pump around
exchanger. The pressure can be controlled by com- mand of the ethylene feed valve. The residence time in
the reactor is fixed by product withdrawal under level
Control. The only left variable is the Catalyst flow
Rate. Once the pressure and temperature fixed, the on- ly way to increase (or decrease) the feed input will be by changing the catalyst rate. Thanks to an automatic catalyst ratio controller the required feed input can be obtained by varying TEAL catalyst flow rate until the feed rate meets the desired value. To increase the yield in 1-Butene, the unconverted ethylene is removed from the product in a distillation tower and recycled back to the reactor in vapor phase. Table 1 indicates operating and design conditions of reactor.
TABLE 1 DESIGN AND OPERATING CONDITIONS OF INDUS- TRIAL REACTOR
Parameter | Value |
Reactor Pressure (Kg/cm2 -g) | 20 |
Reactor Bottom Temperature (oC) | 50.5 |
Pump Around Liquid Temperature (oC) | 44.7 |
Reactor Diameter (m) | 1.8 |
Reactor Height (m) | 12.5 |
Reactor Level (%) | 77 |
3. SIMULATION OF REACTOR
UNISIM was selected as a process simulator for both its simulation capabilities and its ability to incorporate calculations using the spreadsheet tool. The starting step in developing the process simulation was select- ing the chemical components for the process, as well as a thermodynamic model. Additionally, unit opera- tions and operating conditions, plant capacity and in- put conditions must all be selected and specified. UNISIM library contained information for the follow- ing components used in the simulation: Nitrogen, Ethylene, Ethane, 1-Butene, n-Butane, 1-Hexene, 1- Octene and water. Components not available in the UNISIM library were specified using the ‘‘Hypo Manager” tool. Catalyst, TEAL, Active and Active* were all specified in this manner. Specification of a component requires input of a number of properties,
IJSER © 2012
The research paper published by IJSER journal is about Dynamic Simulation of Reactor to Produce 1-Butene by Dimerization of Ethylene 3
ISSN 2229-5518
such as normal boiling point, density, molecular weight, as well as the critical properties of the sub- stance.
Although equation of state models have proven to be reliable in predicting properties of most hydrocarbon
based fluids over a large range of operating condi- tions, their application is limited to primarily non-
polar or slightly polar components. Polar or non-ideal chemical systems are traditionally handled using dual
model approaches. In this approach, an equation of state is used for predicting the vapor fugacity coeffi-
cients (normally ideal gas assumption or the Redlich
Kwong, Peng-Robinson or SRK equations of state,
although a Virial equation of state is available for spe- cific applications) and an activity coefficient model is used for the liquid phase. The non-random two liquid (NRTL) thermodynamic/activity model was selected for use as the property package for the simulation. Since some binary interaction parameters were not available in the simulation databanks, they were esti- mated using the UNIFAC vapor–liquid equilibrium and UNIFAC liquid–liquid equilibrium models where appropriate. Plant capacity was specified at 2000 kg/hr of ethylene.
A kinetic scheme was proposed listed below. This con- sists of four principal reactions with ethylene as a reactant and 1-Butene as a product and hexene-1 as a byproduct.
The catalyst is a titanium compound (LC2253) acti- vated by an alkyl-aluminum (Triethylaluminium or
TEAL).![]()
The Titanium-based active catalyst proceeds with for- mation of active sites by ethylene on a valence IV tita-
nium atom![]()
The active complex proceeds in two steps:
1. Attack of ethylene on active site to give the meta- locyclic intermediate species.
2. Intra-molecular beta-hydrogen transfer, which
gives 1-Butene.![]()
1-Hexene can be formed by combination of 1-Butene with active Complex.
Active Complex + 1-Butene Active Catalyst + 1- Hexene
Based on the experimental data, the kinetic model and its parameters were determined. Using the experimen- tal data and analysis of the experimental reactor, by the proposed model, the following requirements for the CSTR were derived and listed below in Table 2. Rate of reaction can be written as![]()
Where k = A e-(Ea/RT) * T^β
TABLE 2: PARAMETERS FOR REACTION KINETICS
Reaction | A | E(kJ/kgmole) | β |
I | 200000 | 9500 | 1.62 |
II | 5000 | 22000 | 2.37 |
III | 30000 | 20000 | 1.527 |
IV | 10800 | 85000 | 0.2 |
To meet all the requirements mentioned above, The RSTS Team of RIL simulated a bubble reactor by splitting this reactor into five CSTR’s integrated it very meticulously so as to behave as one Bubble type reactor. Due to the high recirculation rate around the
IJSER © 2012
The research paper published by IJSER journal is about Dynamic Simulation of Reactor to Produce 1-Butene by Dimerization of Ethylene 4
ISSN 2229-5518
reactor (the pump around flow-rate which is equal to about 120-150 times the feed Input), the composition can be taken as constant in small sections of reactor effectively working it as a CSTR. The gaseous ethy- lene feed mixed with recycle ethylene from distillation column (downstream of reactor) is fed to the bottom CSTR working as a gas distributor for above reactors. The pump around liquid consisting of catalyst and TEAL is fed to top CSTR working as a liquid distribu- tor for below reactors. Gaseous ethylene is flowing in counter direction to liquid from top that effectively dissolve ethylene in liquid phase.
It is possible to simulate the CSTR with chemical
reactions in both liquid and gas phases by the UNISIM program. This requires the knowledge of the reaction rates in the power-law expression. The matrix of stoi- chiometric coefficients, the reaction rate constants (frequency factor and activation energy) and the ma- trix of all partial orders are employed as input parame- ters. The selectivity of the process depends only on the temperature and the molar ratio Al/Ti in the pump around feed directed into the reactor as volume of the
reactor is taken same as in the plant.
Reactor for production of 1-Butene from gaseous ethylene dissolved in liquid phase was simulated as 5
CSTR each has a diameter of 1.8 m and height of 2.5
m. The temperature profile for each reactor is given below in table 3 and reactor inlet and outlet specifica- tion are listed below in Table 4.
The temperature difference across Reactor-1is maxi-
mum as reaction is exothermic which means maxi- mum reaction is taking place in Reactor-1 ( In case of industrial reactor it is in the top part of the reactor). It is due to the availability of active catalyst sites as TEAL is added just before pump around liquid is in- serted into reactor which ensures that formation of ac- tive sites in top most part of reactor.
TABLE 3: REACTOR TEMPERATURE
Parameters | Reactor-1 | Reactor-2 | Reactor-3 | Reactor-4 | Reactor-5 |
Temperature (oC) | 47.18 | 48.32 | 49.25 | 49.98 | 50.5 |
ΔT (oC) | 2.35 | 1.14 | 0.93 | 0.73 | 0.52 |
TABLE 4: REACTOR INLET AND OUTLET STREAM SPECIFICATIONS
Stream | Feed | Recycle Ethy- lene | LC Cata- lyst | TEAL | Liquid Effluent |
Pressure (kg/cm2-g) | 33 | 22 | 20.6 | 22 | 20.6 |
Temperature (oC) | 32.9 | 31 | 25 | 40 | 50.5 |
Molar Flow (kgmole/h) | 72.8 | 15.4 | 2.3 | 0.2 | 55.3 |
Mass Flow (kg/h) | 2042 | 500.7 | 127.8 | 12 | 2682.5 |
Component fraction | Feed | Recycle Ethy- lene | LC Cata- lyst | TEAL | Liquid Effluent |
Nitrogen | 0.00006 | 0.00831 | 0 | 0 | 0.00278 |
Methane | 0.00002 | 0.0022 | 0 | 0 | 0.00129 |
Ethylene | 0.99948 | 0.69825 | 0.01548 | 0 | 0.29446 |
Ethane | 0.00043 | 0.0104 | 0.0009 | 0 | 0.0037 |
1-Butene | 0 | 0.28069 | 0.98024 | 0.95304 | 0.67087 |
n-Butane | 0 | 0.00014 | 0.0014 | 0 | 0.00008 |
1-Hexene | 0 | 0 | 0 | 0 | 0.02671 |
LC Catalyst | 0 | 0 | 0.00198 | 0 | 0 |
TEAL | 0 | 0 | 0 | 0.04696 | 0.00002 |
H2O | 0 | 0 | 0 | 0 | 0 |
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 6, June-2012
5
ISSN 2229-5518
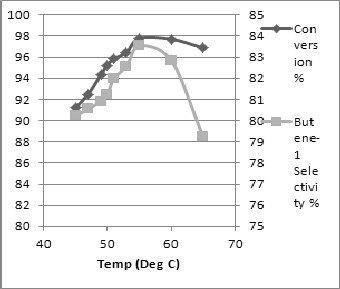
The reaction parameters, which have a manifest influence on the course of the ethylene dime- rization, was found by simulation listed below
The effects of reaction temperature on ethylene con- versions, overall selectivity to total products were in- vestigated. The increase of temperature has a good influence on the dimerisation reaction activity (ethy- lene flow into the reactor) but is detrimental to the se- lectivity (increases the formation of byproducts). The decrease of temperature will affect the rate of conver- sion with the consequence to increase the rate of cata- lyst to keep constant production rate of 1-Butene as illustrated in Fig.2. The ethylene dimerization reaction exhibits a strong dependence on temperature. With the increase in the reaction temperature, the conversion of ethylene and the selectivity to 1-Butene decreases. The decline in the ethylene yield as illustrated in Fig 2 is attributed to the decrease in the monomer solubility at higher reaction temperature. On the other hand, the poor selectivity to 1-Butene could be attributed to the fact that increasing the reaction temperature (at con- stant pressure) provokes an increase in the partial pressure of the formed 1-Butene and a concomitant decrease in the ethylene partial pressure, which leads to the subsequent decrease in the concentration of the latter in the liquid phase. The higher proportion of po- lymer generated at higher reaction temperature could be ascribed to the higher deactivation rate of the active dimerization species leading to the quantitative gener-
ation of the TiIII and TiII species, which are known to
promote polymerization. A similar profile has been observed by Pillai et. Al. [2] in systematic study of titanate catalyst.
Fig 2: The effect of reaction temperature on yield of reaction
The effects of reaction pressure on ethylene conver- sions, overall selectivity to total products and are re- ported at Fig 3. The ethylene dimerization over TEAL and Catalyst is carried out in a liquid phase, the com- position of which is determined by the ethylene pres- sure which, in turn, determines the ethylene conver- sion rate. Higher pressure is associated with an in- crease in the ethylene conversion rate [2]. This obser- vation from Fig 3 could be interpreted as an indication that higher pressure ensures higher activity of the cata- lyst due to an improved diffusion of the monomer through the reaction mixture to the active dimerization sites. This account has been evidenced by the observa- tion of Sergienko et al. [3] of a higher yield of 1- Butene and hexenes at higher pressure. The initial ethylene pressure has apparently no influence on the catalyst’s selectivity to butene-1, but on the other hand an increase in the level of the generated polymer was detected at higher reaction pressure
IJSER © 2012
The research paper published by IJSER journal is about Dynamic Simulation of Reactor to Produce 1-Butene by Dimerization of Ethylene 6
ISSN 2229-5518
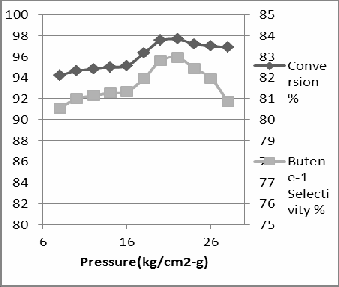
Fig 3 the effect of reaction pressure on yield of reac-
tion
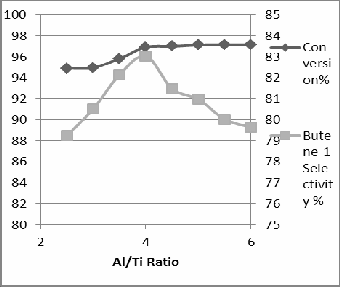
The effect of Al/Ti molar ratios on ethylene conver- sions, overall selectivity to total products was studied and illustrated in Fig 4. The Al/Ti molar ratio is be- lieved to be the most critical factor which determines the course of the ethylene dimerization over the Teal catalyst. This is in agreement with a long-standing as- sumption advanced by Natta [4]which suggested that the reaction is selective to 1-Butene when the Al/Ti molar ratio is < 10, whilst a mixture of dimer, oligo- mer and polymer are produced at higher ratios, with the balance tipped toward the formation of high- molecular-weight polyethylene when Al/Ti > 20. This has later been confirmed by several studies which concluded that ethylene dimerizes selectively to 1- Butene at lower Al/Ti ratios, whereas higher Al/Ti ra- tios are associated with a marked loss in the activity of the dimerisation centers, due to the presence of free AlEt3. An optimal catalyst activity has been detected at A1/Ti range of 3.0-5.0 (subject to the reaction tem- perature).
Fig 4 the effect of Al/Ti on yield of reaction
In the paper, a reactor for the dimerization of ethylene to 1-Butene is proposed. The performance of this reac- tor was simulated by the program UNISIM. The dime- rization is described by a very simple kinetic model, which involves four principal chemical reactions. There is a lack of vapor-liquid equilibrium data in the literature. Therefore, these data were predicted theo- retically using the UNIFAC group contribution me- thod. In the simulation model, the heat exchangers were all optimized. From different stages of the solu- tion of the problem, it can be concluded that the simu- lation program is very flexible and robust and it con- verges to the solution from different starting values of variables estimated. Simulations show that the reactor divided in five CSTR’s could exhibit high selectivity and high yield of 1-Butene. From the practical point of view, the design of the reactor should provide a high interfacial area between liquid and gas phase.
Al: Aluminum
Ti : Titanium
Catalyst: Ziegler type of Alphabutol catalyst
(LC2253),
IJSER © 2012
The research paper published by IJSER journal is about Dynamic Simulation of Reactor to Produce 1-Butene by Dimerization of Ethylene 7
ISSN 2229-5518
TEAL Alkyl aluminum compound,
Active Catalyst Active site formed by catalyst
(LC2253) and TEAL
Active Complex Active site formed by Active Cata- lyst and ethylene
r : Rate of reaction k : Rate constant
A : Frequency factor
Ea: Activation Energy
R : Universal gas constant
β : Unit less coefficient of temperature
[1] A Hennico, J. Leonard, A. Foreatiere, and Y. Giaize, Hydrocarbon.
Process. 69 (1990) 73.
[2] S.M. PilIai, G.L. Tembe, M. Ravindranathan and S. Sivaram, Ind. Eng.
Chem. Res., 27(1988)1971
[3] G.S. Sergienko, V.I. Zhukov and G.P. Belov, Neftekhimiya, 3 1 (1991)
50.
[4] P. Natta, J. Polym. Sci., 34 (1959) 1
IJSER © 2012