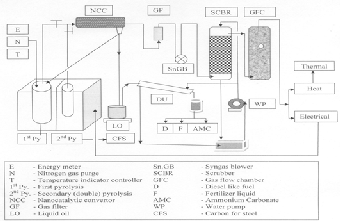
Fig. 1. Process flow
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 61
ISSN 2229-5518
Double Pyrolysis of Chrome Tanned Leather Solid
Waste for Safe Disposal and Products Recovery
Mr. C. Sethuraman1, Mr. Kota Srinivas2, Dr. G. Sekaran3
Abstract— Disposal of chrome tanned leather waste (CTLW) generated in leather processing industries has many environmental implications. This paper presents a double pyrolysis (PI & PII) method to dispose hazardous chrome tanned leather solid waste by converting it into useful products. W hen CTLW was subjected to double pyrolysis, three major products obtained were; (i) an energy enriched combustible gas (32.01% - PI & 42.45% - PII ); (ii) a high fraction condensate (33.32% (PI & PII)) and (iii) a carbonaceous residual ash containing trivalent chromium (34.67%- PI & 24.23% -PII). In addi- tion, 2.73% of liquid fuel, 75.24% of nitrogen enriched liquid and 22.03% distillate tar as sub-products from the high fraction condensate. SEM, GC-MS,
1H & 13C NMR and TGA revealed that the products were free from toxic chromium species and contains both aliphatic and aromatic hydrocarbons. The
residual ash displayed high thermal stability when run under nitrogen atmosphere (simulating 1st pyrolysis). The observations on generation of no toxic compounds upon double pyrolysis, suggests the suitability of this process towards achieving safe disposal of hazardous leather solid waste and to re- cover useful products.
Index Terms— Chromium, Double pyrolysis, Energy recovery, Fuel gas, Leather Waste, Tannery, Zero-discharge
—————————— ——————————
eather industry processes 6.8 million tons of wet salted hides and skins worldwide in a year. It generates about 75-
80% of solid wastes during the process, in which, variety of chemicals used to convert putrescible collagen fibres into non putrescible leather finished products. One ton of wet salt- ed hides would yield only 195 kg of grain and 60 kg of split: a total yield of 255kg of finished leather and the remaining in the form of wastes such as fleshing, trimmings, unusable chrome split, wet blue trimmings, crust leather waste, chrome shaving, buffing dust and finished leather off-cuts [1].
Basic chromium sulphate (BCS) is the most widely used tanning material for converting putrescible collagen fibres into non putrescible leather matrix. Only 60% of chromium salts applied for tanning process react with the raw materials and the rest of chromium salts disposed along with the waste ma- terial and also into the waste water [2]. Amongst the waste, CTLW is a proteinous fine particulate solid waste impregnated with chromium, synthetic fat, oil, tanning agents and dye chemicals. About 2–6 kg of CTLW is liberated as a solid waste per ton of skin/hide processed [3].
————————————————
• Mr. C. Sethuraman1 is currently pursuing Ph.D degree program in Madras University at CSIR-CLRI, Environmental Technology Division, Chennai, India PH- 04422541061. E-mail: csramcsio@gmail.com
• Mr. K. Srinivas2 is Chief Scientist and Scientist in Charge of CSIR-CSIO Chennai Unit, Chennai, India, PH-04422541061. E-mail: sriniwaskota@gmail.com
• Dr. G.Sekaran3 is Chief Scientist and Cluster Chairman, CSIR- CLRI, Chennai, India, PH-04424452941. E-mail: ganesansekaran@gmail.com.
The energy content of tannery leather solid waste is more than
50% in comparison to hard coal, is normally 20 MJ/kg in dry
basis [4]. The chromium content in solid leather waste (wet
blue leather), is approximately 30g kg−1 (w/w) [5]. Brazilian
Environmental Council (CONAMA) classified chromium con-
taining leather waste material as a category-one waste, one of
the most dangerous and harmful wastes if discarded into the
environment without any further treatment [6].
Chrome containing leather waste is carcinogenic in nature and it causes clinical problems like respiratory tract ailments, ulcers, perforated nasal septum, kidney malfunction [7] and lung cancer [8]. Because of this, such a material needs a special disposal, which is very expensive [9]. In the absence of any economically viable technology to dispose the solid leather waste, land co-disposal, thermal incineration and anaerobic digestion methods are currently being practiced [10]. Current practising methods of disposing these wastes have the follow- ing disadvantages:
The available landfill sites rapidly reach their total capacity and the authorization of new sites becomes difficult [11]. The improper and the manual handling and transfer of leather waste in open vehicles create unhygienic conditions. Disposal of waste in low- lying areas without proper liners allow the leachate to mix with ground water causing water contamina- tion.
It has been reported [12] that during thermal incineration at
800°C, Cr3+ was converted to Cr6+ by 40%. Thermal incineration
causes serious air pollution problems due to emission of toxic
hexavalent chromium (Cr6+), halogenated organic compounds,
poly aromatic hydrocarbons etc. into environment. The major
species formed from Cr3+ during thermal incineration of solid
waste are Cr2 (SO4 )3(s ), CrOCl2(g) and Cr2 O3(s ) which later trans-
formed into Cr6+ [13]. Hexavalent chromium (Cr6+) is mobile in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 62
ISSN 2229-5518
the environment and is highly toxic. It can penetrate the cell wall and exert its noxious influence in the cell itself, being also
TABLE 1
a source of various cancer diseases [14]. At short-term expo- CHARACTERIZATION OF CTLW
sure levels above the maximum contamination level, Cr6+ caus-
es skin and stomach irritation or ulceration. Long-term expo-
sure at levels above the maximum contamination can cause
dermatitis, damage to liver, kidney circulation, nerve tissue
damage and death [15, 16]. Air pollution is created by odour
nuisances and the generation of green house gases from most
of the landfill sites. Investments cost on anaerobic digestion
plant is very high and also it does not provide a solution for
zero waste disposal.
An earlier study on leather wastes suggested that pyrolysis experiments on leather wastes at 450 °C and 600 °C using fixed bed reactor loaded with 50-60 g, yielded the charred residue and ammonium carbonate besides gas and oil products [17]. It was stated that the carbonaceous residue (chars) resulted was in the range 37.5%-48.5% and their calorific value was in the range 4300-6000 kcalkg, suitable for use as solid fuel. The heat content of the gas was not tested and concluded that waste leather is a useful recycling resource and the conversion of wastes into activated carbon and fuels may be recognized as an attractive approach and further works are necessary before large-scale application.
The encouraging statement of Yilmaz et al (2007) was the lead for the present study and based on the above summarized drawbacks and the necessity on management of chromium containing leather solid wastes. The present study focussed the management of chromium containing leaterher wastes through double pyrolysis. Like incineration, pyrolysis is also a thermal process that uses high temperatures to break down the organic waste without the participation of oxygen. The advantage of pyrolysis over combustion is reduction in CO2 which play a vital role in green house effect. In single pyrolysis system, percentage of residual carbonaceous material (40%) needs additional treatment system, which is a major drawback observed from our earlier experiments.
Hence, a double pyrolysis approach was used in this study to assess the mass of the residual carbonaceous material, gases and value added products. The by-products obtained from the process were further subjected to chemical and analytical methods.
CTLW was characterised for moisture content, volatile matter, carbon, ash, chromium, fat, protein content, calorific value etc., using standard procedures and the results are given in table-1.
# By Difference
The main parts of the double pyrolysis system are i) furnace for raw feed and residual carbon for second pyrolysis ii) reac- tor stills iii) microprocessor based temperature indicator con- troller iv) digital energy meter v) condenser vi) gas filter vii) syngas blower viii) water circulation pump ix) scrubber x) gas flow chamber vi) distillation unit as shown in the following process flow diagram (Fig. 1). The outer chamber of the fur- nace was fabricated using mild steel with necessary reinforce- ment for mechanical rigidity. The outer dimensions of the fur- nace were length, 650mm; breadth, 650mm and height,
450mm. The reactor still of first pyrolysis was made up of SS316 grade with inner diameter, 150mm; height, 300mm; with wall thickness, 5mm. The secondary pyrolysis reactor still was also made up of SS316 grade with inner diameter, 80mm; height, 200mm; with wall thickness, 5mm.
The waste material loaded in the reactor still heated in the box type furnace using A1 grade kanthal heating coils, tem- perature sensor, chrome/alumel (“K” type) with single phase
230V A.C. operated by micro processor based temperatue indi- cator controller using thyristors. This electrically operated furnace works based on resistive heating principle. Due to the resistive property of the Kanthal coils, power fed to the ele- ments are converted to heat energy. This heats up the inner chamber of the furnace where the CTLW samples are placed as per the temperature programming set up to 800 °C. The vola- tile matter generated from the reactor enters into trimetallic nano-catalytic converter (NCC) to enhance the combustion properties of the gas.
The liquid oil collected from NCC goes to distillation unit where the by-products viz. i) thiol containing diesel like fuel, ii) ammonium containing fertilizer liquid, iii) ammonium car- bonate salt is produced. The gas which is free from liquid oil passes through the gas filter, scrubber, gas flow chamber and got cleaned. The cleaned gas from gas flow chamber can be
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 63
ISSN 2229-5518
used for both thermal and electrical applications. The volatile matter removed residual taken out from the first pyrolysis re- actor still then transformed into secondary pyrolysis reactor still and heated upto 900ºC to remove the left over volatile matter present in the residue after first pyrolysis.
Fig. 1. Process flow
Considering the input quantity of 2 kg load and material phys- iochemical properties, the batch type double pyrolysis gasifica- tion system was designed as per the specification given below:
Input quantity in mass, m = 2 kg
Specific heat of chrome tanned
leather, 10% moisture, Cp = 0.35 cal/g/°C
Density of CTLW = 0.38 g /cm3
Volume of reactor = 2000 / 0.38
= 5263 cm3
Internal diameter of reactor = 15cm
Length of the reactor = 5263 / (3.142 x 7.5 x 7.5)
= 29.77 cm
Thickness of reactor vessel, t = 3mm
Weight of the reactor vessel = 10.30kg
The heat to rise the 10.30g SS 316 grade material from ambient
(30°C to 800°C), its specific heat is 0.38 cal/g/°C
= 10.30 x 0.38 x (800-30)
= 3013.78kcal
Interms of power, P = 3013.78 /860
= 3.5 kW (3500W)
This power can be met with single phase (i.e. Voltage at 230V),
the required current to be passed through the resistive heating
coil is = P / V
= 3500 / 230
= 15.21A
Resistance of the coil, R = 230 / 15.21
= 15.12 Ω
Electrical heating coils, 12 numbers, each 300W; 2Ω resistance,
12 coils kept inside the ceramic tubes, connected in series (8
coils in service and 4 coils kept as spare), temperature sensor
insulated with glasswool is shown in Fig. 2 (a, b). The heating
capacity of 8 coils is 2400W. Depents upon the requirement of
rate of heating all 12 coils could be connected in series to
achieve 3600W.
Fig. 2 Fabrication of pyrolysis furnace a) connected with 12 coils, b) temperature sensor with glasswool insulated ceram- ic tubes.
The elemental compositions interms of Carbon, Hydrogen, Ni- trogen and Sulphur (CHNS) of raw CTLW, residual carbon ob- tained from first and second pyrolysis, liquid oil were deter- mined using Elementar-Vario Micro Cube analyser. Thermo- Gravimetric Analysis (TGA) was carried out to assess the weight loss as a function of temperature under nitrogen atmos- phere using Universal TGA Q50 V20.6 Build 31. The loss of mass of the residual ash also carried out under air atmosphere using TGA Q500 V20.10 Build 36 to study the effect of air in mass reduction.
Scanning Electron Microscopy (SEM) analysis was carried out using Hitachi S-3400N to determine the surface morpholo- gy of the samples with high resolution. Energy Dispersive X- ray analysis (EDAX) was carried out on raw CTLW, residual ash and liquid oil to determine the elemental composition. Fou- rier Transform Infrared (FTIR) spectroscopy analysis was car- ried out using Thermo Nicolet, model 330 to determine the functional groups present in the samples. The hexavalent chromium (Cr6+) content in the residual carbon of first and sec- ondary pyrolysis, and in liquid oil were determined calorimet- rically by alkali digestion using NaOH, Na2 CO3 , MnSO4 in the presence of phosphate buffer (K2 HPO4 and KH2 PO4 ). The Cr total content was determined by acid digestion using strong oxi- dising agent and followed by reaction with diphenyl carbazide.
The CTLW was characterised for moisture content, 5.50%; pro- tein content, 80.63%; and pH, 4.67. The composition of CTLW as per ultimate analysis was Carbon, 55.31%; Hydrogen, 7.86%; Nitrogen, 12.56%; Sulphur, 4.68%; Oxygen, 8.54%. The proxi- mate analysis showed the volatile matter, 60.30%; ash, 7.58% and fixed carbon, 26.62%. The density of CTLW was 260kg/m3 and Gross Calorific Value was 4246kcal/kg.
The CTLW sample (weight 2.83mg) was heated in a platinum
pan from 30 to 800°C at the rate of 20°C/min. The TGA spec-
trum of CTLW under nitrogen atmosphere (Fig. 3a) shows that
the weight loss was only 5% upto 222.23°C. There was a
steady decrease in weight of the sample by 56.22% upto
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 64
ISSN 2229-5518
481.52°C and this may be attributed to the elimination of sur- face bound water molecules and volatile organic compounds from the matrix. There was a further reduction in mass by
8.61% on heating from 481.52°C to 789.83°C due to the thermal decomposition/ volatilization of the peptide compounds in which carbon constitutes the back bone of the chain and at- tached with functional groups viz hydrogen, nitrogen contain- ing group, carboxylic acid group. The side attached to the main carbon chain volatized at higher temperature which re- sulted in the reduction in mass by about 65% at 800°C. The TGA of the CTLW suggests that the volatile organic com- pounds were destructed upto 500°C using the combined oxy- gen of 26.77% in it. The residual ash resulted during destruc- tive distillation of CTLW under nitrogen atmosphere (simulat- ing 1st pyrolysis process) was 35.17%. The TGA of residual ash under nitrogen atmosphere (simulating 1st pyrolysis process) shows that the material was thermally stable upto 791.80°C since the mass of unburned residual ash was 81.69% (Fig. 3b), this suggests that very little change in mass of residual ash alone would be resulted under 1st pyrolysis condition. The TGA of residual ash obtained under double pyrolysis (Fig. 3c) shows that the mass of unburned residual ash was 45.96% at
800°C. It indicates that double pyrolysis could reduce the mass of the unburned residual ash from 81.69% to 45.96% which may be due to the delinking of peptide bonds and for- mation of carbon attached gaseous compounds at high tem- perature. The TGA of condensate oil (Fig. 3d) shows that the solid residue left at 296.45°C was 11.68% and it was reduced to
3.586% at 791.06°C, indicating that the condensate oil has more than 96% of volatile components.
Fig. 3. TGA spectra of a. CTLW under nitrogen atmosphere, b. residual ash under 1st pyrolysis, c. residual ash under double pyrolysis and d. condensate oil
The SEM image of CTLW (Fig. 4, a) shows the isolated collagen fibres. This is due to the presence of fat liquors that surround- ed the fibres causing their separation. SEM analysis of residual ash presented in figure (Fig. 4, b) shows that 1st pyrolysed state of collagen fibres as separated carbon slabs. SEM image of double pyrolysis residual ash (Fig. 4, c) indicates that the car- bon slabs were reduced to the flakes of ash with inorganic chromium spreading as white granules over it. However, the SEM analysis of distillate as shown in figure (Fig. 4,d) indicates that the condensate oil is not pure homogeneous, it is admixed with other substances.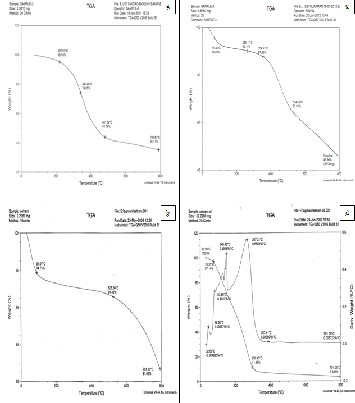
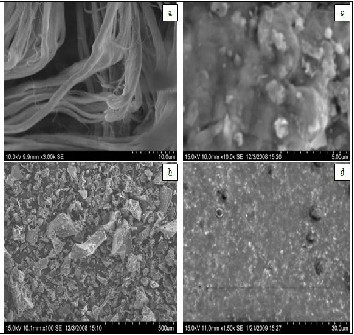
Fig. 4. SEM analysis of a. CTLW, b. residual ash under 1st py- rolysis, c. residual ash under double pyrolysis and d. conden- sate oil
Hexavalent chromium (Cr6+) was analysed as per ISO 17075 by UV-VIS-Spectrophotometer on residual ash obtained from 1st pyrolysis and double pyrolysis and found that the valule was below detectable limit.
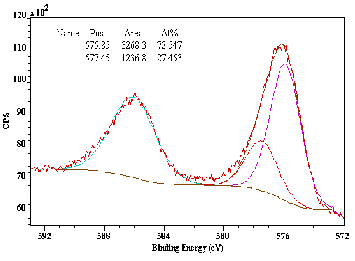
The residual ash sample ubtained under double pyrolysis process sent for hexavalent chromium (Cr6+) analysis con- firmed its absence, since no peaks at 579 eV was recorded in the XPS analysis as shown in Fig. 5.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 65
ISSN 2229-5518
The number of chemically equivalent carbon atom can be ob- tained from the C-13 NMR. The signals in C-13 NMR spec- trum from 12 to 35ppm indicate the presence of aliphatic hy- drocarbon. The observed no signals from 40 to 65ppm reveals the absence of alcoholic and halogen groups. The signals from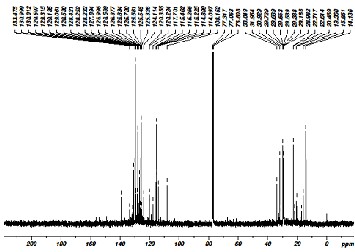
110 to 140ppm show the presence of unsaturated hydrocar- bons. The signals from 125 to 150ppm show the presence of carbon in aromatic rings. Since, no signals beyond 150ppm show the absence of carboxyl and carbonyl groups in the com- pounds. The aliphatic and aromatic hydrocarbons attached with sulphur and absence of oxygen enhances the fuel proper- ties and it can also be used as additive for detecting the gas leakage.
Fig. 5. XPS spectra of residual ash ubtained under double pyrolysis
In order to study the nature of the protons and carbons in the compounds of the condensate oil, 1H and 13C NMR were tak- en. Proton NMR spectrum is shown in Fig. 6 and Carbon NMR is shown in Fig. 7. Since, signals are found in wide range from 1 to 8ppm indicates the presence of mixture of com- pounds rather than single compound. The total no. of protons identified in the spectrum is 77. The downfield shift from 1 to
1.8ppm reveals presence of electro negative atoms in aliphatic mixture. The electronegative atom can be halogen, oxygen or sulphur. Since, there are no signals above 8ppm that shows the absence of oxygen functional groups (i.e., acid and carbonyl groups). The raw material i.e., CTLW used in this pyrolysis process contains no halogen, it can be suggested that the shift has happened due to the presence of sulphur compounds. There are 58 aliphatic protons and 19 aromatic protons.
Fig. 7. Carbon NMR (13C)
The concentration of the major compounds analysed by CG- MS is given in table no. 2.
TABLE 2
CONCENTRATION OF COMPOUNDS IN LIQUID OIL
Name of the compounds | Concentration in mg/L |
Benzene Ethyl benzene 4-Isopropylbenzene Naphthalene Toluene o- Xylene m & p- Xylene | 736.87 393.27 29.73 172.81 1376.91 287.27 199.3 |
Fig. 6. Proton NMR (1H)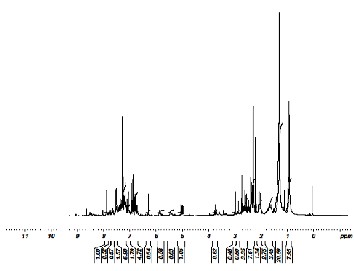
The above table shows that the compound was the mixture of aromatic carbon which increases the fuel property of the liquid oil. Since the number of carbon atoms in the individual com- pound was not very high, the fuel property of the condensate oil seems to be more. Heavy hydrocarbons are not present in the mixture, if present will suppress the fuel property.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 66
ISSN 2229-5518
It was found the formation of ammonium carbonate salt when the condensate oil was distilled at 100°C by qualitative meth- od. The compound was found to have ammonium as the cati- on as the salt was dissolved in water. The anion was found to be carbonate since brisk effervescence was observed when di- luted HCl was added to the salt. Hence, the generated salt from the distilled liquid was ammonium carbonate. The digi- tal microscopic image of ammonium is shown in Fig. 8.
Fig. 8 Images of ammonium carbonate derived from dis- tilled bottom layer liquid of leather solid waste
The following table 3 gives details of mass and balance of the products obtained from pyrolysis and douoble pyroly- sis process.
MASS AND ENERGY BALANCES OF PRODUCTS RESULTED FROM 1 KG OF CTLW UNDER PYROLYSIS AND DOUBLE PYROLYSIS PROCESSES
The clean combustible gas and other useful by-products generated from pyrolysis and double pyrolysis method are shown in the following Fig. 9.
Fig. 9. Useful products derived from hazardous leather solid waste: 1. fuel gas, 2. condensate oil, 3. pyrolysis residual ash 4. double pyrolysis residual ash, 5. distillate condensate oil formed with two layers; top layer is diesel like fuel and bottom![]()
1st Pyrolysis
Double pyrolysis
layer is nitrogen enriched liquid, 6 ammonium carbonate, 7.
(I) (II) remnant distillate tar & residual and 8. carbon residual ash
Yield % GCV
% GCV
containing trivalent chromium (Cr
3+)
(kJ/kg) (kJ/kg)
Gas fuela | 32.01 | 9529.12 | 42.45 | 11490.97 | 5. CONCLUSION |
Residual | 34.67 | 7865.25 | 24.23 | 5903.40 | |
ash Condensate | 33.32 | --- | 33.32 | --- | The present study is the first kind of attempt on management of chromium containing leather solid wastes through double |
Liquid fuelb | 2.73 | 380.63 | 2.73 | 380.63 | pyrolysis for its safe disposal while recovering useful products without conveting the oxidation state of trivalent chromium |
Nitrogen | (Cr3+) into hexavalent chromium (Cr6+) form in the residual ash. | ||||
enriched liquidc | 75.24 | --- | 75.25 | --- | A double pyrolysis process yielded a high energy content combustible renewable gases with minimum (24.23 wt. %) re- |
Distillate tar 22.03 --- 22.03 --- sidual mass. The generated gases find wide industrial applica-
a By difference, b,c Generated out of 333.27g condensate. GCV
of residual ash under pyrolysis 22688kJ/kg and under double
pyrolysis 24360kJ/kg. GCV of liquid biofuel 41328 kJ/kg.
tions and can considerably replace the thermal energy re-
quirement. The liquid by-products can also find immense ap-
plications due to the presence of thiol groups and nitrogen
content. The residual carbon ash which contains trivalent
chromium (Cr3+) can be used in steel manufacturing industries
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 67
ISSN 2229-5518
This research was supported by Council of Scientific & Indus- trial Research, CSIR New Delhi (A clean fuel from hazardous leather solid waste – OLP 0198 & SUSTRANS Net Work Pro- ject). The authors are thankful to CSIR and the Director- CSIO
& Director - CLRI for their active support.
REFERENCES
[1] J. Buljan, G. Reich, and J. Ludvik, “Mass balance in leather pro cessing”, US/RAS/92/120, Regional programme for pollution control in the tanning industry in South East Asia, 9 August., 2000
[2] S. Swarnalatha, K. Ramani, A. Geetha Karthi, and G. Sekaran, “Starved air combustion-solidification/stabilization of primary chem- ical sludge from a tannery”, Journal of Hazardous Materials B137,
304-313, 2006
[3] G. Sekaran, K.A., Shanmugasundaram and M. Mariappan, “Characteization and utilization of CTLW generated by the leather industry”, Journal of Hazardous Materials B 63 53-68, (1998).
[4] K. Fela, K.W. Ciurowa, M. Konopka, and Z. Wozny, “Present and prospetive leather industry waste disposal”, Polish Journal of Chemi- cal Tecnology, 13,3, 53-55, 2011
[5] D.Q. Lima, L.C.A. Oliveira, A.R.R. Bastos, G.S. Carvalho, J.G.S.M.
Marques, J.G. Carvalho and G.A. De Souza, “Leather Industry Solid Waste as Nitrogen Source for Growth of Common Bean Plants”, Re- search Article-Applied and Environmental Soil Science, Volume 2010
Article ID 703842, 7 pages doi:10.1155/2010/703842, 2010.
[6] Conama, “Conselho Nacional Do Meio Ambiente,” Brasília: Resolução no. 357, 1. Diário Oficial 17.05.2005 Ofício no 88.351/83,
2005.
[7] A.H.G. Love, “Chromium – biological and analytical considertions, D. Burrows (Ed.)”, Chromium metabolism and toxicity, CRC Press, Boca Raton, FL, p. 1, 1983.
[8] A. Leonard and R.R. Lawverys, “Carcinigenicity and mutagenity of chromium”, Mutat. Res.76 227-239, 1980.
[9] R. Aravindhan R., Madhan B, Rao J. R, Nair B. U, and Ramasami T, “Bioaccumulation of chromium from tannery wastewater: anap- proach for chrome recovery and reuse,” Environmental Science and Technology, vol. 38, no. 1, pp. 300–306, 2004.
[10] F. Sampere, J. B. Martinez, M.L. Font-Montesinos, and M.C. Sabater-
Lilo,“Characterisation of tannery wastes-comparison of three leacha- bility tests”, Journal of Hazardous Materials 54, 31-45, 1997.
[11] D.W. Kirk, C.C.Y. Chan, and H. Marsh, “Chromium behaviour during thermal treatment of MSW fly ash”, Journal of Hazardous Materials B90, 39-49, 2002.
[12] A. Simoncini, Voice, Leather Survey, p. 210, 1988.
[13] J. Chen, M.Y. Wey, B. Chiang, and S. Hsieh, “The simulation of hexa- valent chromium formation under various incineration conditions”, Chemosphere 36(7), 1553-1564, 2003.
[14] C. Barnowski, N. Jakubowski, D. Stuewer, and J.A.C. Broekaert, “Spe- ciation of chromium by direct coupling of ion exchange chromatog- raphy with ICP-MS. At. Spectrom, 1155 (12), 1155-1161. Doi:10.1039/a702120h, 1997.
[15] F. Katz and H. Slem, “The biological and environmental chemistry of
chromium”, (pp.51-58), New York: VCH, 1994.
[16] J. Kotas and Z. Stasicka, “Chromium occurrence in the environment and methods of its speciation”, Environmental polltion,107(3), 263-
283, 2000.
[17] O. Yilmaz, I.C. Kantarli, M. Yuksel, M. Saglam, J. Yanik, “Conversion of leather wastes to useful products, Journal of Resources”, Conserva- tion and Recycling 49, 436-448, 2007.
AUTHORS BIOGRAPHY

He has received his M.Sc., M.Tech., MBA. degrees from Madurai Kamaraj University, Devi Ahilya University and Pondicherry University in 1994, 1996 and 2008 respectively. He has regis- tered for Ph.D programme under University of Madras. He is currently a Senior Scientist, Central Scientific Instruments Organ- isation, Chennai. His areas of interest include energy manage- ment, energy from wastes, renewable energy, solar photovoltaic systems, energy audit, waste heat recovery, biofuels and clean development mechanism.
First Author, C. Sethuraman
He has received his M.Sc (Tech) and M.S. degrees from
Nagarjuna University, Guntur and BITS, Pilani in 1980 and
1994 respectively. He is currently a Chief Sceintist, heading
Energy Management group and Scientist in Incharge of
CSIO Chennai Unit of Central Scientific Instrumentation
Organisation, India. His areas of interest include
embedded systems based instrumentation, bio sensors,
sensor characterization, modeling, energy conservation

and energy
management.
Second Author, K. Srinivas

He has received his M.Sc and Ph.D degrees from University of Madras in 1978 and 1990 respectively. He is currently a Chief Scientist, heading the Department of Engineering Technology, Central Leather Research Institute, Adyar, Chennai, India. He is an honorary professor of Anna University in the faculty of leather technology. His areas of interest include heterogeneous catalysis applied to oxidation of organics and inorganics in wastewater and in gaseous stream using carbon based heavy metal doped cata- lysts.
Third Author, Dr. G. Sekaran
IJSER © 2013 http://www.ijser.org