International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 1
ISSN 2229-5518
Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples
S.O. Ajayi, B.O. Odesanya, A.A. Fashina & J.O. Ajayi
Abstract - The transient nature of the nitrite ion and its toxicity make on-site determinations preferable to ensure actual values are obtained for analytical data integrity. A simple, compact and cheap field kit for the on-site determination of nitrite in water and environmental samples is presented using a method of colour development based on mixed reagents – (N-(1-napthyl) ethylenediamine dihydrochloride and sulphanilamide) with standard nitrite solutions used to calibrate the field kit before determination of the nitrite in water samples.
Results indicate the method is sensitive, selective and reproducible and would provide a reliable method for the determination of nitrite in water and environmental samples on-site.
Key Words: Field Kit (development), mixed reagent, (N-(1-napthyl) ethylenediamine dihydrochloride, sulphanilamine, calibration graph, on-site determination of nitrite.
Nitrite ion, NO2
—————————— ——————————
nitrite levels on-site for environmental management and
afety. Recently, a simple and portable colorimeter using a
formed during the decomposition of organic matter. Nitrite is present in wastewaters, effluents from sewage treatment plants, water distribution systems and natural waters and has been shown to be highly toxic to all aquatic organisms22,20,34. Dutt and Davis10 have shown that concentrations between 0.1 – 0.2 ppm are enough to poison fishes.
The World Health Organization (WHO) gives 0.2ppm as
the highest desirable limit and 3.0ppm as maximum permissible limits17 while the United States public health service puts 0.06 ppm as the maximum permissible limits27,28 for nitrite concentrations in potable water. Methods exist for the determination of nitrite in water, blood plasma, food and soil
29,31,11,15,24,25,27,36,6,16,2,12,30,19,5,28,10,26,35,33,32,13. The Greiss-Ilosvic
azo-dye method is the most popular. It is a sensitive and
standard method which is adequate for monitoring nitrite concentrations in aquaculture, potable water production and environmental management. The laboratory procedure for the method is well developed3 only that the sample requires effective sample preservation. The field option for the method has not received extensive research.
Due to the toxicity of the nitrite ion7,8,23,9,22,4,21,14,1 and its transient nature as a result of air oxidation or bacterial activity10, there is a need to develop a field kit to determine
Prof S.O. Ajayi & Dr B.O. Odesanya are of the College of Pure and Applied Sciences, Caleb University, Imota. Lagos State while Mr. A.A. Fashina & Mr. J.O. Ajayi are of the University of Ibadan, Nigeria. All correspondence to skipaodes@yahoo.com
red-green-blue light emitting diode was developed for on- site determinations33. More work still needs to be done in this direction.
This investigation aimed at a simple, portable, reliable and sensitive field kit for the determination of nitrite in water on-site based on the well established Greiss-Ilosvic standard method. The thrust of the investigation is to minimize equipment and reagents requirements for field work without compromising the method, its accuracy and sensitivity.
EXPERIMENTAL
The developed field kit consists of a tungsten light source with three filters for red (710nm), yellow (580nm) and green (520nm) regions for wavelength selection and a Light Dependent Resistor (LDR) which serves as a sensor preceding the 1cm2 sample compartment. The LDR converts the light that emerges from the sample compartment into analog-digital voltage with the resistance of the LDR varying with the concentration of the sample solution. This variation of the LDR’s resistance is measured as absorbance.
Reagents ( APHA Method 4500, 2000)3
2.5g of sulphanilamide was weighed and dissolved in a mixture of 25mL hydrochloric acid and 150mL deionised water. The solution was then made up to the mark in a
500mL standard flask.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 2
ISSN 2229-5518
0.5g of N-(1-naphthyl) ethylenediamine dihydrochloride was weighed and dissolved in 500mL deionised water in a standard volumetric flask.
1.232g of sodium nitrite was weighed into a 1000mL
standard flask, 1mL of chloroform was added to preserve the solution before it was made up to the mark. The ensuing nitrite solution was standardized with 0.05M KMnO4 using sufficient ferrous ammonium sulphate solution as reductant.
Traditional laboratory procedure for Nitrite analysis by azo-dye method using sequential addition of reagents
50mL of standard nitrite solution was measured and the pH of the solution was brought to 7.0 by the addition of a phosphate buffer solution of pH 7.0. 1mL of sulphanilamide was added to this solution and left for 8 minutes for complete reaction after which 1mL of N-(1- naphthyl) ethylenediamine dihydrochloride solution was added and mixed thoroughly. The solution was left to stand for 10 minutes before the absorbance was taken with a UV-Vis spectrophotometer at 520nm with the mixed reagent as blank.
Mixed Reagent Method
In developing the field kit, two methods of colour development were considered: (i) normal and (ii) mixed reagents. The normal method involved the sequential addition of sulphanilamide to nitrite samples for diazotization before N-(1-naphthyl) ethylenediamine dihydrochloride is added to get the highly coloured azo dye chromophore. However, the reagents for the mixed reagent method (sulphanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride) were mixed in a 1:1 ratio to obtain one reagent. Analytical procedure is essentially the same except that 1 mL of mixed reagent was used for each 50 mL sample.
RESULTS AND DISCUSSION
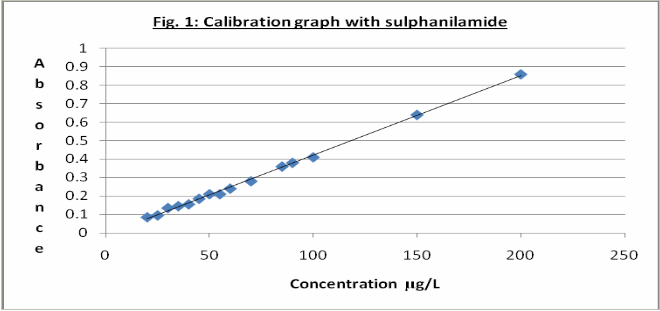
There are many combinations of reagents for the azo-dye colour development in the determination of the nitrite ion. This work considered two of the foremost combinations: (a)sulphanilamide and N-(1-naphthyl)ethylenediamine dihydrochloride and (b) sulfanilic acid and N-(1-naphthyl) ethylenediamine dihydrochloride. Figures 1 and 2 show the calibration graphs obtained using the two sets of reagents. The results show that the sulphanilamide- N-(1-naphthyl) ethylenediamine dihydrochloride combination with standard nitrite solutions gave a linear calibration graph with a regression coefficient of 0.997. The sulfanilic acid- N- (1-naphthyl) ethylenediamine
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 3
ISSN 2229-5518
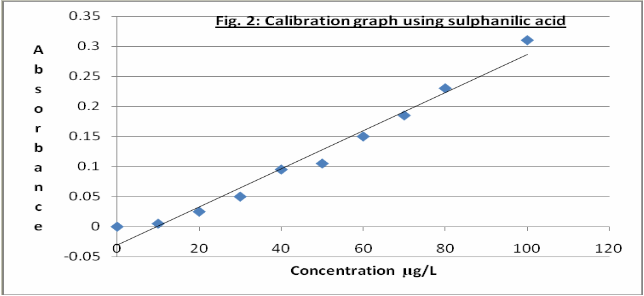
dihydrochloride-standard nitrite solutions also gave a fairly linear graph but the absorbance values were much lower. The better linearity, intensity and stability of colour formed made us adopt the sulphanilamide- N-(1-naphthyl) ethylenediamine dihydrochloride combination as the reagents of choice for colour formation in this study. A
comparison of the absorbance values obtained using the method of sequential addition (Normal) and mixed reagents are given in Table 1.
Concentration of nitrite, (ppb) | Absorbance values at 520nm Normal method Mixed reagents method | |
25 | 0.090 | 0.090 |
50 | 0.145 | 0.150 |
75 | 0.250 | 0.230 |
100 | 0.300 | 0.280 |
R2 | 0.979 | 0.992 |
Table 1: Absorbance values using Normal and Mixed reagents methods
The F-test value of 0.8364 indicates a 2-tail probability that the variances between the two sets of absorbance values are not statistically different. A 2-tailed, homoscedastic T-test gave a value of 0.8954 implying an insignificant difference between the 2 sets of absorbance values. Therefore, in a bid to minimize the number of glassware required for field
measurements, reduce the volume of reagents required for colour development and achieve the objective of producing a field kit that is simple and portable, the mixed reagent method is adopted for these determinations.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 4
ISSN 2229-5518
Sensitivity test for adopted method
In order to test the sensitivity of the adopted mixed reagent method and reproducibility of analytical data, 0.025 ppm and 0.05 ppm nitrite solutions were prepared and the
absorbance values determined using the mixed reagent with different volumes but same ratio of mixed reagent to standard nitrite solution.
The results are given in Tables 2 and 3.
Volume of mixed reagent plus standard nitrite solution | Absorbance at 520nm* |
2.0ml of mixed reagent plus 2.5ml of 0.025ppm nitrite solution made up to 50ml in std. flask | 0.080 |
1.0ml of mixed reagent plus 1.25ml of 0.025ppm nitrite solution made up to 25ml in std. flask | 0.075 |
0.5ml of mixed reagent plus 0.625ml of 0.025ppm nitrite solution made up to 12.5ml | 0.080 |
*values are averages of triplicate readings
Volume of mixed reagent plus standard nitrite solution | Absorbance at 520nm* |
2.0ml of mixed reagent plus 5.0ml of 0.05ppm nitrite solution made up to 50ml in std. flask | 0.155 |
1.0ml of mixed reagent plus 2.5ml of 0.05ppm nitrite solution made up to 25ml in std. flask | 0.145 |
0.5ml of mixed reagent plus 0.125ml of 0.05ppm nitrite solution made up to 12.5ml | 0.155 |
*values are averages of triplicate readings
Table 2 shows the absorbance values obtained with different volumes of 0.025 ppm standard nitrite solutions and mixed reagent. Reduction in volume of the standard nitrite solution and volume of reagent made insignificant difference in absorbance values. A similar trend was observed with standard 0.05ppm nitrite solution (Table 3). Although this relative consistency of absorbance values is expected due to the same dilution factor being used, it is also an indication that the mixed reagent method gives
reproducible results and can be a sensitive method of colour development. In a bid to confirm the sensitivity of the method, the volume of the mixed reagent was reduced to one and two drops respectively. The drop(s) of the mixed reagent were fed directly into the 1.0ml cuvette using a 2ml syringe with the volume of standard nitrite or sample solution added afterwards before stirring. The absorbance values are taken at least in triplicates. The results of absorbance taken at 520nm are presented in Table 4:
Concentration of std. nitrite solution (ppm) | 1 drop of mixed reagents plus 1.5ml std. nitrite solution* | 2 drops of mixed reagents plus 1.5ml std. nitrite solution* |
0.5 | 0.95,0.96,1.02,0.95 (0.97) | 1.10,1.05,1.10,1.00 (1.06) |
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 5
ISSN 2229-5518
0.4 | 0.95,0.95,1.0 (0.97) | 1.02,1.02,1.0 (1.05) |
0.3 | 0.78,0.80,0.77,0.83 (0.80) | 0.78,0.80,0.83,0.83 (0.81) |
0.2 | 0.58,0.60,0.58,0.60 (0.59) | 0.56,0.62,0.62,0.60 (0.60) |
0.1 | 0.36,0.37,0.35,0.35 (0.36) | 0.40,0.41,0.38,0.39 (0.395) |
0.075 | 0.16,0.175,0.20 (0.18) | 0.18,0.19,0.175 (0.182) |
Plots (figures 3 and 4) indicate a linear relationship up to
0.4ppm solution. A linear regression plot gave R2 values of
0.9707 and 0.9728 for one and two drops of mixed reagent up to 0.4 ppm also giving values of 0.9193 and 0.9377 respectively up to 0.5 ppm. The results indicate that the method is reproducible and of enough sensitivity for use as an analytical method. Statistical examination of the results using a 2-tail homoscedastic T-test gave a value of 0.8519 showing that using 1 or 2 drops of mixed reagent does not
significantly affect the absorbance values. However, for the purpose of this study, 2 drops of mixed reagent was used.
Adoption of Field Procedure
A field procedure was adopted for this investigation thus: Cuvette in colorimeter, add 1 mL sample, 2 drops pH 7.0 buffer solution plus 2 drops mixed reagent from 2 mL syringe. Mix with syringe and measure at 520nm.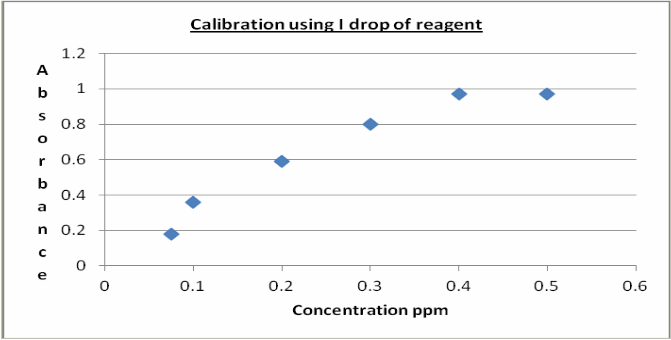
Figure 3: Calibration using 1 drop of reagent
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 6
ISSN 2229-5518
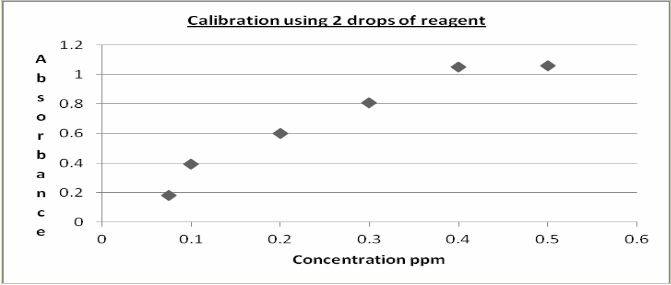
Figure 4: Calibration using 2 drops of reagent
Stability test for the Mixed Reagent
In a bid to determine the effects of environmental factors on the reagents before use, calibration graphs were prepared using:
(a) Mixed reagent preserved in a refrigerator before
use
(b) Mixed reagent kept at room temperature before
use
(c) Sulphanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride kept separately in a refrigerator and mixed just before use and
(d) Sulphanilamide and N-(1-naphthyl)
ethylenediamine dihydrochloride preserved
(e) separately at room temperature and mixed just before use.
Absorbance readings were taken for each concentration and category of reagents for 25 days. A comparison of the values for (a) and (b) for 25 days showed no significant difference. A similar pattern was observed for categories (c) and (d). There was a very low standard deviation (n = 25) making us to conclude that the mixed reagent is stable for nitrite determinations. The results are presented in Tables 5 and 6.
This established the feasibility of the method for nitrite determinations.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 7
ISSN 2229-5518
Concentration ( g g m) | Rea g e n t Con d it ion s Reagent at room Temperature eagent preserved in Refridgera dition of reagents at room Temp on of reagents preserved in Re | Absorbance 0.06 0.06 0.05 0.07 | ||
0.03 | Mixed Mixed R Sequential ad Sequential additi | Rea g e n t Con d it ion s Reagent at room Temperature eagent preserved in Refridgera dition of reagents at room Temp on of reagents preserved in Re | (A) tor (B) erature (c) fridgerator (D) | Absorbance 0.06 0.06 0.05 0.07 |
0.06 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.24 5 0.24 0.26 5 0.24 5 | ||
0.09 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.27 0.25 0.26 0.26 | ||
0.12 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.40 5 0.43 0.415 0.43 | ||
0.15 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.43 5 0.44 0.43 5 0.43 5 | ||
0.18 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.4 7 0.4 7 0.48 5 0.49 5 | ||
0.21 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.6 0.62 5 0.61 0.60 5 | ||
0.24 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.68 5 0.67 5 0.68 5 0.66 5 | ||
0.27 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.78 5 0.78 5 0.76 5 0.80 5 | ||
0.3 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature (c) Sequential addition of reagents preserved in Refridgerator (D) | 0.93 0.94 0.93 5 0.96 5 |
Table 5: Effects of temperature variation on Absorbance for method
*All readings are averages of duplicate readings
IJSER !b) 2011
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 8
ISSN 2229-5518
Concentration (ppm) | Reagent Conditions | Absorbance* | Standard deviation |
0.03 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.059 0.0562 0.063 0.053 | 0.0147 0.0136 0.0417 0.0135 |
0.06 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.2488 0.2434 0.2484 0.244 | 0.0123 0.0494 0.0202 0.0156 |
0.09 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.2736 0.2607 0.2766 0.267 | 0.0109 0.051 0.0207 0.0141 |
0.12 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.3826 0.3822 0.3731 0.3718 | 0.0253 0.034 0.0426 0.0479 |
0.15 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D)) | 0.4416 0.4446 0.4418 0.444 | 0.0137 0.0118 0.0184 0.0166 |
0.18 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.476 0.4762 0.4802 0.4774 | 0.0168 0.0124 0.0303 0.033 |
0.21 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.5618 0.5594 0.5606 0.5508 | 0.0232 0.0241 0.0438 0.0295 |
0.24 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.6612 0.6616 0.6732 0.6728 | 0.015 0.0257 0.0389 0.0202 |
0.27 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) SSequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.769 0.7704 0.7796 0.772 | 0.0194 0.023 0.0411 0.0206 |
0.3 | Mixed Reagent at room Temperature (A) Mixed Reagent preserved in Refridgerator (B) Sequential addition of reagents at room Temperature ( C ) Sequential addition of reagents preserved in Refridgerator (D) | 0.9286 0.9194 0.9266 0.9254 | 0.0167 0.0232 0.0189 0.0153 |
Table 6 : Stability ckeck for method over 25 days.
(* Absorbance readings are average values over 25 days.)
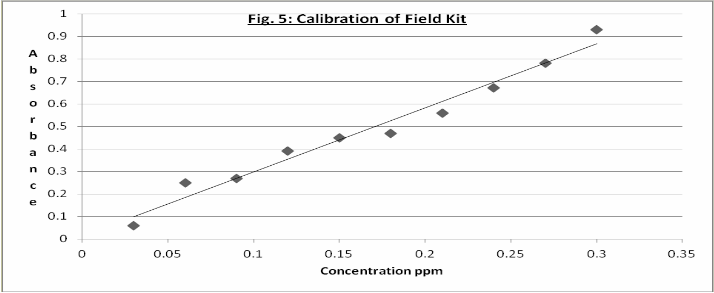
Translation of method to Field Kit Calibration of Field Kit and Determination of Nitrite concentration in Water samples
To enable the field kit be used, a calibration graph using
Various nitrite concentrations – 0.03, 0.06, 0.09, 0.12,
0.15, 0.18, 0.21, 0.24, 0.27 and 0.3 ppm- was prepared. The resulting calibration curve is given in figure 5. A correlation between concentration of nitrite and absorbance was established with a regression coefficient (R2 = 0.989) indicating its suitability for use for calibration.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 9
ISSN 2229-5518
The procedure was adopted for use in the determination of nitrite concentration in samples obtained from Awba dam and along the river flowing into it. The mixed reagents method was also applied for nitrite determinations in
different fish ponds. The results are shown compared with those obtained using the sequential method and are given in Tables 7 and 8.
Location along the river and Awba Dam | Absorbance at 520nm*(Normal method) | Absorbance at 520nm* (Mixed Reagents method) | Concentration of Nitrite µg/mL |
Point 1 | 0.11 | 0.11 | 0.035 |
Point 2 | 0.11 | 0.11 | 0.035 |
Point 3 | 0.16 | 0.16 | 0.052 |
Awba Dam | 0.095 | 0.095 | 0.030 |
Samples | Absorbance at 520nm* (Normal method) | Absorbance at 520nm* (Mixed Reagents) | Concentration of Nitrite µg/mL |
Surface fish pond | 0.072 | 0.075 | 0.0165 |
Concrete fish pond | 0.066 | 0.065 | 0.0129 |
Concrete fish pond with recycling facility | 0.035 | 0.035 | 0.0024 |
(* Average of triplicate readings)
CONCLUSION
A field kit and mixed reagent method for the determination of nitrite has been developed. It has been successfully applied for the on-site determination of nitrite in Awba
Dam, along the river flowing into the dam and in some selected fish ponds.
The method is sensitive, reproducible and stable and can be used in the determination of nitrite in water samples for routine analysis.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 10
ISSN 2229-5518
nitrite by concentration of azo-dye on an ion-exchange resin. Analytical Chimica Acta, 56, 233 – 240
REFERENCES
59 13 – 27
2. AL HATIM, A. A. (1990): Spectrometric determination of nitrite in aqueous solution by diazotization coupling method with p-aminobenze- N-(1-napthyl)-ethylene. International Journal of Environmental Analytical Chemistry, 38(4), 617-622.
3. APHA method 4500 for Nitrite. 2000.
4. ARILLO, A., GAINO, E., MARDIOCCO, C., MENSI, P. and SCHENNONE, G. (1984): Biochemical and ultrasound effects in rainbow trout: liver hypoxia as the root of the acute toxicity mechanism. Environmental Research, 34, 135-254.
5. BADEA, M., AMINE, A., PALLESCHI, G., MOSCANE, D., VOLPE, G. and CURULL, A., (2001): New electrochemical sensors for detection of nitrites and nitrates. Journal of Electro-analytical Chemistry, 66, 509.
6. BASHIR, W. A., FLAMERZ, S. and SAAD, K. I. (1983): Spectrophotometric determination of nitrite in aqueous solution by diazotization coupling method with orthaniline acid-resorcinol. International Journal Environ. Anal. Chem, 15, 65-71
7. CAMERON, J. N. (1971): Methaemoglobin in Erythrocyte of rainbow trout. Comparative Biochemistry and Physiology, 40, 743-749.
8. CRAWFORD, R. R. and ALLEN, G. H. (1977): Seawater inhibition of nitrite toxicity to Chinook salmon. Transaction of the American Fisheries Society, 106. 105-109.
9. DE FLORA, S. and ARILLO, A. (1983): Mutagenic and DNA damaging activity in muscle of trout exposed in vivo to nitrite Cancer Letters, 20, 147-155.
10. DUTT, J. and DAVIS, J. (2002); Current Strategies in nitrite detection and their application to field analysis. Journal of Environmental Monitoring, 4, 465 – 471.
11. EL TARO, W. and AKIHI, H. (1971): Spectrophotometric determination of traces of
12. GUAGHAN, L., HONG, J and DARDEN, S. (1997): Determination of trace nitrite by anodic stripping voltammetry, Food chemistry, 59, 583 -687
13. HIROMI, T.; AZUZA, T.; MAKOTO, M.; TAMAO, O.;
HIROKI, H.; TOMONARI, U. and KIN-ICHI, T. (2006): Liquid core waveguide spectrophotometry for the sensitive determination of nitrite in river water
samples. Analytical Sciences. 2, 1017 – 1019.
14. HOFFER, R. and GATUMU, E. (1994): Necrosis of trout
retina (Oncorhynchus myskiss) after sub lethal exposure
to nitrite. Achieves of Environmental Contaminant and
Toxicology, 26, 119-123.
15. HUEY, D. W. SIMCO, B. A. and CRISWELL, D. W. (1980): Nitrite-induced methaemoglobin formation in channel catfish. Transactions of the American Fisheries Society, 109, 558-562.
16 .IBRAHEEM, B. B. and BASHER. W. A. (1987): Spectrophotometric determination of nitrite in aqueous solution by the diazotization-coupling method with p aminoacetophenone-N-(1- Naphthyl) ethylenediamine. Inter J. Environ Anal. Chem., 20.159-166)
17. IPAN. (2005): Preadmission workshop. Institute of Public
Analysts, Appendix (II)
18. JENSEN, F. B. (2003): Nitrite disrupts multiple physiological functions in aquatic animals. Comparative Biochemistry and Physiology, Part A. 134, 9-24.
19. JIE, N., YANG. D., JIANG Q., ZHANG, Q. and WEI, L. (1999): A fluorescence quenching method for the determination of nitrite with indole. Microchemical Journal, 62(3), 371- 376
20. KROUPOVA, H., MACHOVA, J, and SVOBODOVA, Z (2005): Nitrite influence on fish- A review, Veterinary Czech, 50 (11), 461-471.
21. LEWIS, W. W. and MORRIS, D. P. (1986); Toxicity of
nitrite: a review. Transaction of the American Fisheries
Society. 115, 183-195.
22. MARGIOCCO, C., ARILLO, A., MENSI, P. and SHENONE, G. (1983): Nitrite bioaccumulation in salmon gairdneri. Rich and haematological consequences. Aquatic Toxicology, 3, 261-270
23. MENSI, P., ARILLO, A., MARGIOCCO, C. and SHENONE, G. (1982): Lysosomal damage under nitrite intoxication in rainbow trout. Comparative Biochemistry and Physiology, 73, 161-165.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, The research paper published by IJSER journal is about Development of a Field Kit Using The ‘Mixed Reagents Azo-Dye Method’ For The Determination of Nitrite In Water And Environmental Samples 11
ISSN 2229-5518
24. NAGARAJA, P., KUMAR, M. S. H., RAN-GAPPA, K.S. and BILWA, L.M. (1998): A new spectrophotometric reagent for the determination of nitrite in polluted water. Oriental Journal of Chemistry, 14, 55-58.
25.NAKAMURA, m. and MUZUKA, t. (1983): Spectrophotometric determination of nitrite in waste water with 4, 5 – dihydroxycoumarin. Analytical Letter, 16(A11),
811 – 819.
26. NORIKO, H.; MISAKI, K.; and SHIGERU, T. (2003):
Micro-phase sorbent extraction for trace analysis via in situ sorbent formation; application to the spectrophotometric determination of nitrite in environmental waters. Analytical Sciences, 19, 239-243.
27. PADMARAJAIAH, N.; MATTIGHATTA, S.; HEMANTHA, K.; (2001): Highly sensitive N-(1-Naphthyl) ethylene diamine method for the spectrophotometric determination of trace amounts of nitrite in various water samples. Inter. J. Environ chem. 39-48.
28. PADMARAJAIAH, N.; MATTIGHATTA, S.;
HEMANTHA, K., KANCHUGARAKOPPAI., S. RANGAPPA (2001): Dapsone and Iminobenzyl as novel reagents for the spectrophotometric determination of trace amounts of nitrite in water samples. Analytical Sciences, 17,
439 – 442.
29. SALTZMANN, B. E. (1954): Colorimetric micro- determination of nitrite in the atmosphere. Analyst Chem.,
26, 1949.
30. SATAKE, M. and GEN-FENG. W. (1997): Spectrophotometric determination of nitrite in natural waters using diazotization-coupling method with column pre-concentration on naphthalene supported with ion-pair
of tetradecyldimethlbenzylammonium and iodide.
Fresenius Journal of Analytical Chemistry, 357(4), 433-438.
31. SAWICKI. E., STANLEY, T. W., PFAFF, J., and D’AMICO, A. (1963): Comparison of fifty two spectrophotometric methods for the determination of nitrite. Talanta, 10,641-655.
32. SHIZUKO, H., SATHRUGNAN, K. and TASUKU, K. (2004): Portable flow-injection analyzer for nitrite and nitrate in natural water. Analytical Sciences 10, 567-569.
33. SUZUKI, Y.; TERUTOMI, A.; HIROYUKI, K.; KUWABARA, T.; KAWAKUBO, S. and IWATSUKI, M. (2004): A simple and portable colorimeter using a red- green-blue emitting diode and its application to the on-site determination of nitrite and Iron in the river-water. Analytical Sciences, 20, 975-977.
34. SVPBPDPVA, Z., MACHOVA, J. POLESZCUK, G. HUDA, J., HAHCKOVA, J., KROUPOVA, H. (2005): Nitrite poisoning of fish in aquaculture facilities with water re- circulating systems – three case studies. Acta Veterinaria Brno, 74, 129– 137
35. WEN, X. H. and KANG, T. (2004): Determination of nitrite using sensors based on nickel phthalocyanice polymer modified electrodes. Talanta, 62(2), 351-355.
36. WU, Q., FENG, L. and PENG, F. (1983): Spectrophotometric determination of microamount of nitrite in water and soil. Shaanxi monitoring station- Environmental Proto, Xian People’s Rep-China. Talanta,
30(5), 374-376.
IJSER © 2011 http://www.ijser.org