The aerial roots of Rhaphidophora aurea
intertwined over Lawsonia inermis and Areca catechu
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 59
ISSN 2229-5518
P. Arulpriya and P.Lalitha
Abstract- The proximate composition and chemical constituents of the aerial roots of Rhaphidophora aurea (Linden ex Andre) intertwined over Lawsonia inermis (MM) and Areca catechu (MB) were evaluated using standard methods. The results of proximate composition of MM and MB showed the following; total ash (10-13%), acid soluble ash (32-34%), water soluble ash (71-72%), dry matter (99%), moisture (5-12%), fiber (88-90%), fat and wax (6-7%). It also contained phytoconstituents like alkaloids, flavonoids, phenolics and terpenoids, quaternary alkaloids and N-oxides. These results directly impede the drug effect, activity, stability, potency and the side effects of drug. The results of the study portray the phytochemical quality of the sample which affects its pharmacological properties.
Keywords- Proximate composition, phytoconstituents, Rhaphidophora aurea
—————————— ——————————
IJSER
Proximate analysis in plant samples (root, leaf, stem or whole plant) gives valuable information about its chemical composition and is helpful to get access to the quality of the plant. It provides information on moisture content, ash content, volatile matter content and fixed carbon etc. Ash is the inorganic residue remaining after water and organic matter have been removed by heating, which provides a measure of the total amount of minerals within the plant. Minerals are not destroyed by heating and they have a low volatility as compared to other plant components. Total ash may vary with in wide limits for specimen of genuine drugs due to variable natural or physiological ash. Ash value gives us an idea of the mineral matter contained in a plant. Measuring it is essential, because mineral matter might be a cause for pharmacological effect [1].
The aerial roots of Rhaphidophora aurea
intertwined over Lawsonia inermis and Areca catechu![]()
Corresponding author: Dr.P.Lalitha, Assistant Professor (SS) of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore- 641043, Tamil Nadu, India. Email- goldenlalitha@gmail.com Mobile No: 09842292614
Co –author: P.Arulpriya, Research Scholar, Department of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore-641043, Tamil Nadu, India.
revealed phytoconstituents like alkaloids,
flavonoids, tannins, terpenoids, steroids, anthraquinone [2]. The solvent extracts of this plant has been reported by us in our previous paper to possess wound healing [3], larvicidal [4], antimicrobial [2] and antioxidant activity [5].
Der-Jiun Ooi have reported the variation of Proximate composition and phytochemical properties with agroclimatic conditions, humidity and species of the plant [6]. The ethno pharmacologists, botanists, microbiologists and natural product chemists in the world over today are constantly in search of medicinal efficiency of plants and their phytochemicals, since the reported data so far available on plants are comparatively meager before the vast number of plant population. Subsequently the approach of this paper is to investigate the phytochemical and proximate composition of the aerial roots of Rhaphidophora aurea intertwined over Lawsonia inermis and Areca catechu.
Aerial roots of Rhaphidophora aurea intertwined over Lawsonia inermis (MM) were collected from Coimbatore District and Areca catechu (MB) were collected from Palakkad District and the botanical identification was carried by Dr G.V.S.Murthy, Joint Director, Botanical survey of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 60
ISSN 2229-5518
India, Coimbatore. The plant authentication number is BSI/SC/5/23/09-10/Tech- 1534.
Accurately weighed pulverized MM (1 g) and MB (1g) and placed into a pre-weighed china dish. It was placed in an oven (1050C) overnight (12 hr) and cooled thereafter in a dessicator. The dishes were removed from desiccator and weighed thrice. The percentage of the dry matter was calculated as:
(Weight of dish +
Weight of dried sample) – Weight of dish![]()
Weight of sample before drying
Weight of dry sample![]()
= ×100
Weight of sample before drying
Weight of fresh sample – Weight of dry sample![]()
crucible, dried on a hot plate and ignited to constant weight. The residue was allowed to cool
in a desiccator for 30 minutes and then weighed. The acid insoluble ash percentage was calculated as below and expressed as mg per g air-dried sample.
Weight loss of ash ×100![]()
Weight of ash (500 mg) taken
Calculated quantities of ash (500mg) was treated with 15ml, boiled for 5 minutes and filtered. The insoluble ash matter was collected in a crucible, washed with hot water and ignited in a silica for 15 minutes at 320-3500C in an oven. The water soluble ash percentage was calculated as bellow and expressed as mg per g of air-dried sample.
Calculation
Weight loss of ash×100![]()
Weight of ash (mg) taken
IJSER
Weight of fresh sample
The pulverized MM and MB were analyzed for total ash, acid insoluble ash and water soluble ash according to standard procedures as follows:
The pulverized MM (6g) and MB (6g) were weighed accurately and poured into a previously ignited and dry silica crucible. The material was evenly spread and ignited via Bunsen flame for 30 min. The crucible was placed in a Muffle furnace and the temperature was gradually increased to 6000C till it became white, indicating the absence of carbon, then placed the dishes in a desiccator for cooling and weighed. The total ash percentage was calculated as bellow and expressed as mg per gram air-dried sample.
(Weight of crucible + ash) –
Weight of crucible![]()
Weight of sample (MM/MB)
Calculated quantities of ash (500 mg) were treated with 2N HCl (15ml) covered with a watch glass, boiled gently for 5 min and filtered. The insoluble matter was collected and washed with hot water until the filtrate became neutral. The filter paper was then transferred to a pre-weighed
Pulverized MM (5g) was taken in a round bottomed flask, added 150 ml of methanol: water (4:1) and refluxed for 12 hours, cooled and filtered using Whatmann filter paper no: 41. Similar procedure was adopted for MB extraction also. The filtrates were refrigerated for further use. The plant residue was used for determining the fiber content.
The plant residue obtained from the above extraction was further extracted with 125 ml of ethyl acetate (12 hours soaking + 2 hours refluxing) and filtered. The plant residue consisting of plant fiber and the filtrate was used for determining the fatty matter.
The filtrate from the above procedure was allowed to evaporate on a water bath (450C) and placed in a desiccator for cooling. The weight of the residue gives the quantity of fat and wax.
The filtrate obtained from the extraction was allowed to evaporate to 1/10th of the volume at
700C on a water bath, acidified with 2M H2 SO4 and extracted with chloroform (75 ml). The procedure was repeated till the chloroform layer was colourless. Collected the both aqueous and chloroform layer in a beaker and allowed the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 61
ISSN 2229-5518
chloroform layer to evaporate and placed in a desiccator for removing its moisture content, then the chloroform layer was weighed and consists of phenolics and terpenoids.
The aqueous layer obtained from the above procedure was neutralized with 2M NaOH and extracted with 60 ml of 3:1 chloroform: methanol, followed by extraction with 40ml of chloroform. The procedure was repeated till the chloroform layer become colourless. Both the aqueous and chloroform layers were separated using a separatory funnel. The chloroform was distilled off and the alkaloid fraction obtained was weighed.
Aqueous layer obtained from the above procedure was allowed to evaporate on a water bath at 700C, dried and weighed to get the yield of quaternary alkaloids.
Extracts (1ml) were treated with chloroform, acetic anhydride and added drops of H2 SO4 . A dark green ring was indicative of terpenoids
The extracts were heated with sodium nitrite, add H2 SO4 solution diluted with water and add excess of dilute NaOH and observed for the formation of deep red or green or blue colour.
The extract was treated with neutral ferric chloride and observed for dark coloured solution.
The proximate analysis is directly related to stability of drug which directly influences drug potential, drug action and drug effects. Drug solubility has its own importance in
pharmaceuticals and medical sciences because
IJSER
Pulverized (2g) plant material was
extracted with 20 ml of 80% aqueous: methanol via
soaking (12 hrs) and filtered. The residue and filtrate were allowed to evaporate and weighed to get the yield of flavonoids.
Dragendorff’s reagent (1ml) was added to one ml of extract. An orange-red precipitate indicates the presence of alkaloids.
Mayer’s reagent (1ml) was added to one ml of extract. Cream colored precipitate indicates the presence of alkaloids.
About 0.5ml of each extract portion was dissolved in ethanol, warmed and then filtered. Three pieces of magnesium chips was then added to the filtrate followed by few drops of conc. HCl. A pink, orange, or red to purple colouration indicates the presence of flavonoids.
A small amount of extract was treated with lead acetate. The formation of white precipitate was indicative of the presence of flavonoids.
specific, non-specific and physicochemical
interactions like lipid solubility, osmomolarity membrane penetration of drugs etc. depend on these results. These results directly hamper the drugs effect, drug activities, stabilities of drug and drug potency also the side effects of the drug depend upon transport of drug across cell membranes and through blood in the body [14].
The pulverized MM and MB were
evaluated for its total ash value, acid insoluble ash, water soluble ash, dry matter, moisture content, fiber, fat and wax and the results are given in Tables 1, 2 and 3.
Ash value is useful in determining
authenticity and purity of the sample. Generally ashes are two types, one is physiological ash and other one is non-physiological ash. Physiological ash is the ash inherent in the plant due to biochemical processes and the non-physiological is contaminants from the environment. These may be carbonates, phosphates, nitrates, sulfates, chlorides and silicates of various metals which were taken up from the soil [10].
The non-physiological ash component of
the total ash could be reduced by rinsing the fresh plant material several times in clean water before drying and processing for medicinal use
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 62
ISSN 2229-5518
contamination and chemical degradation [14] as it provides a medium for many reactions to occur. The less moisture content does not promote microbial and chemical contamination. These results are best for drug transport and drug receptor interactions are controlled force in dilute solutions, which increases potency, drug action and drug effect.
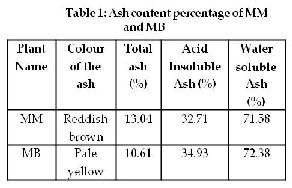
It is well apparent from Table 1 that both the plant
materials (MM and MB) contain a minimum percentage of ash (13.03 and 10.61 respectively) content. The MM ash percentage is slightly higher compared to that of MB. Generally higher ash content implies that the plant has good or high organic components in it and low ash content implies a rather low inorganic or mineral constituent. The high ash content is a reflection of the mineral contents preserved in the plant
materials [11]. The ash content is generally
The determination of dry matter is useful to find the volatile nutrients of the plant. Table 3 shows
IJSER
recognized as a measure of quality for the
assessment of the functional properties of foods
[12].
The acid-insoluble ash value measures the
amount of silica, especially siliceous earth, present
in the drug plant [11]. The physiological ash gets dissolved in the dilute acid while some of the non- physiological ash remains undissolved [13]. Acid insoluble ash content of MM (32.71%) and MB (34.93%) shows that the small amount of inorganic compound is insoluble in acid and as a result the plant may be readily digested and absorbed when consumed. Higher value of acid insoluble ash indicates the higher digestibility when the plant is consumed.
Water soluble ash content values from the table 1 indicates that, the plant has less contamination with metal ions [12]. The percentage of the water soluble ash content of MM is 71.58% and MB is 72.38%. Water soluble ash content was higher compared to acid insoluble ash. These results show that both MM and MB may contain less metal ions.
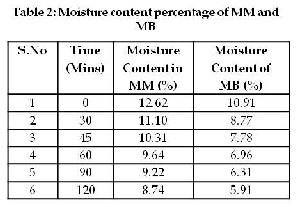
The moisture content percentage from
table 2 indicates that the less moisture content was
observed in both MM and MB. This low moisture content implies the stability of natural product, high moisture content tends to promote microbial
MM and MB to contain a high percentage of dry
matter. Generally plants can lose all the volatile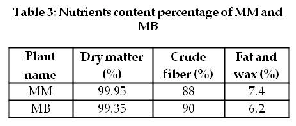
nutrients at 100°C (oven). Volatile nutrients of importance are essential oils (camphor, menthol) and short chain fatty acids (propionic, acetic, butyric, etc.) also can be significant with some feeds. Drying samples at 100°C can volatilize a few of these materials, resulting in greater humidity (lower dry matter) values.
High fiber content is obtained from MM
and MB (Table 3), MM yield 88% and MB yield
90%. The richest sources of dietary fiber are
employed in the treatment of diseases such as obesity, diabetes, cancer and gastrointestinal disorders [15]. The fibrous nature of the pulp could as well offer protection against certain diseases such as cancer of the colon and increase bowel content transit time.
MM and MB revealed good yield (table 4)
of alkaloids, flavonoids, phenolics and terpenoids, quaternary alkaloids and N-oxides. These
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 63
ISSN 2229-5518
phytoconstituents were confirmed through phytochemical screening tests.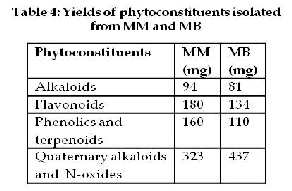
The aim of isolation of secondary metabolites is to analyze the mechanism of action of useful compounds to accelerate the invention of modern medicine. Most of the secondary metabolites are used as healthful agents, flavor, colour, stimulant, hallucinogens and etc.
The plant derived phenolic and terpenoids have great remedial importance that is most vital
cluster of organic compounds. The presence of
as a strong antioxidant, free radical scavenging and metal collecting capacity [22].
Proximate evaluation and its composition will provide more information on nutrient composition and chemical content of the plant. Also this evaluation may helpful to do the isolation of phytochemicals and pharmacological studies. High contents of total fat, total ash and crude fiber contents of Xylopia aethiopica is particularly recommended for women who have newly given birth as a tonic in Ivory Coast [16]. For the present study, both the MM and MB has exposed high content of ash, fat and fiber content. Hence this may have pharmaceutical importance.
The proximate evaluation of MM and MB confirms that the aerial root of Rhaphidophora aurea twined over Lawsonia inermis and Areca catechu contain less moisture content indicating that the sample plant can be stored for a period of time without spoilage and it will not be susceptible to
microbial growth. The low fat content reveals low
IJSER
phenolic and terpenoids in plants can encourage further research for possible new drugs leads [17].
Alkaloids are natural plant constituent,
have basic character and it contains a minimum of
one atomic number 7 atom in an exceedingly hetero-cyclic ring which express varied biological activity. Alkaloids are imperative chemical compounds that give a rich reservoir for drug discovery. Numerous alkaloids which are segregated from natural herbs show anti- metastatic and anti-proliferation effects on different types of cancers both in vivo and in vitro [18].
The extractability of quaternary alkaloids
as in N-oxides tends to cause a significant challenge to natural product Chemists, particularly within the resource - restricted countries. This can be owing to their high level of polarity and therefore a major affinity to the aqueous-acid phase sometimes utilized within the common extraction method [19].
Flavonoids form a large class of significant naturally occurring bioactive compounds [20]. Flavonoids have reported anti-allergic, anti- bacterial, anti-inflammatory, anti-viral and anti- neoplastic activity [21]. Many of these alleged effects have been linked to their known functions
total inorganic mineral, which makes is useful for
therapeutic purpose. The obtained secondary metabolite from MM and MB suggests that the plant might be of industrial (pharmacy) and medicinal importance. ACKNOWLEDGEMENT
The authors thank the authorities of the Avinashilingam Institute for Home Science and Higher Education for Women (Etd. u/s 3 of UGC Act 1956), Coimbatore -43 for providing the facilities to carry out this research work. REFERENCES
[1] D. Nair Lethika, K. Sar Santosh, Arora Arun, Mahapatra Deepak, “ A comparative study on proximate analysis conducted on medicinal plants of Chhattisgarh”, Research Journal of Chemical Sciences, Vol. 2(9), pp.18-21, Sept. 2012.
[2] P. Lalitha, K.A. Arathi, Shubashini K Sripathi, S.
Hemalatha, P. Jayanthi, “Antimicrobial activity
and phytochemical screening on an ornamental foliage plant Pothos aurea”, Alfa universal, vol. 1(2), pp.1-07, June. 2010
[3] P. Arulpriya, P. Lalitha, “The wound healing potential of aerial roots of Rhaphidophora aurea (Linden ex Andre) climbed over Lawsonia inermis”, Asian Journal of Pharmaceutical and Clinical Research, vol. 6(1), pp.132-135, Dec.2013.
[4] P. Arulpriya, P. Lalitha, N. Aarthi, “Larvicidal and pupicidal effect of ethanolic extract of the aerial roots of Rhaphidophora aurea intertwined over four different host trees against Culex quinquefasciatus
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 64
ISSN 2229-5518
say”, Research Journal of Pharmaceutical, Biological and Chemical Sciences, vol.4(1), pp. 18-27, Jan-Mar.
2013.
[5] S. Hemalatha, P. Lalitha, P. Arulpriya, “Antioxidant activities of the extracts of the aerial roots of Pothos aurea (Linden ex Andre)”, Der Pharma Chemica, Vol.2 (6), pp. 84-89. 2010.
[6] Der-Jiun Ooi, Shahid Iqbal, Maznah Ismail, “Proximate composition, nutritional attributes and mineral composition of Peperomia pellucida L. (Ketumpangan Air) grown in Malaysia”, Molecules, vol. 17, pp.11139-111452, Sep.2012.
[7] J.B. Harborne, “Phytochemical methods. A guide to modern techniques of plant analysis”, 2nd ed, Chapman and Hall, 1973, London.
[8] C.K. Kokate, “Practical Pharmacognosy”, 4th ed, Vallabh Prakashan, 1994, Delhi.
[9] A. Sofowara, “Medicinal plants and traditional medicine in Africa”, John Wiley and sons Ltd,
1982, Chichester.
[10] O.F. Kunle, “Phytochemical and microbiological studies of the leaf of Lippia multiflora Mold, FAM. Verbanaceae.”, A PhD Thesis of Ahmadu Bello University – Zaria, pp.127-129, 2000.
[11] B.S. Antia, E.J. Akpan, P.A. Okon, I.U. Umoren,
[14] P. Rajesh, A.H. Ganorkar, J.S. Atloye , O. Bansod, S. Deshmukh, D.A. Pund, “Proximate analysis of root, stem and leaves of Cissus Quadrangularis Linn”, Int. J. Chem. Sci, vol. 10(2), pp. 855-860,
2012.
[15] L.G. Saldanha, “Fibre in the diet of U.S. Children: Results of national surveys”, Pediat, vol. 96, pp.
994-996, Nov.1995.
[16] H.M. Burkill, “The useful plants of West Africa”,
Royal Botanical Gardens, vol. 1, pp. 11-20, 1985.
[17] W.C. Evans, “Trease and Evans Pharmacognosy”,
15th Edition, Elsevier, India., pp. 27, 46, 183-184,
289-291, 411-413, 434, 485-486, 2002.
[18] J.J.Lu, J.L.Bao, X.P. M. Chen, Y.T. Huang, Y.T Wang, “Alkaloids isolated from natural herbs as the anticancer agents”, Evid Based Complement Alternat Med, Vol. 2012, pp. 485042, Sep. 2012.
[19] N. Oguegbulu, N. Edwin, Uche I Fidelia, “Extraction and detection of the alkaloids of the bark of Alstonia boonei plant (family: apocynaceae) by the sulphonephthalein dye-stuff ion-pair complextion at pH 5.58”, Journal of Pharmaceutical and Biomedical Sciences, vol. 15(15), pp. 1-4, 2012.
[20] F.D. Costa, G.G. Leitão, “Strategies of solvent system selection for the isolation of flavonoids by
IJSER
“Nutritive and anti-nutritive evaluation of sweet,
potatoes (Ipomoea batatas) leaves”, Pak. J. Nutr, vol.
5, pp.166-168, 2006.
[12] A.J. Patel, R. Patel Jatin, P. Macwan Carol, A. Patel Mayuree, K. Soni Arun, “Pharmacognostical and proximate analysis of leaves of Borreria Hispida”, Asian Journal of Biochemical and Pharmaceutical Research, vol. 2 (1), pp.157-161, Apl.2011.
[13] E.J. Shellard, “Exercises in the evaluation of drugs and surgical dressings” 1st Edition, Pitman Medical Publishing Co. Ltd, London, pp. 81-95.
1958.
countercurrent chromatography”, J Sep Sci, vol.
33(3), pp. 336-47, Feb. 2010.
[21] L. Alan, N.D. Miller, “Antioxidant flavonoids: structure, function and chemical usage”, Alt. Med. Rev, vol. 1, pp. 103–111, 1996.
[22] T. Nakayama, M. Yamada, T. Osawa, S. Kawakishi
, “Suppression of active oxygen-induced
cytotoxicity by flavonoids”, Biochem Pharmacol.
Vol. 45(1), pp. 265-7, Jan.1993.
IJSER © 2013 http://www.ijser.org