Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 1
ISS N 2229-5518
Decolourisation of Synthetic Dyes by Agricultural
Waste- A Review
Neetu Sharma, D.P. Tiwari, S. K. Singh
Abstract— Decolourisation of waste water has now become a major problem for the treatment plants in various industries. Many industries use synthetic dyes to colour their products such as textiles, rubber, paper, plastics, leather, cosmetic, food etc. Nearly 10-15% of synthetic textile dyes, used yearly are lost to waste streams and about 20% of these losses enter the enviro n- ment through effluent from waste water treatment plant. Numerous techniques were used in the recent past for decolourisation of dyes. Among them adsorption technique has got maximum potential for the removal of dyes. Adsorption being a physical process, in-expensive and less time consuming, is widely accepted. It is evident from last 20 -25 years that many researchers have studied the feasibility of low cost adsorbents derived from natural material, i ndustrial material, agricultural waste and bio- adsorbents and resulted in innovative approach in this area. The current research is focussed on the need to develop an efficient adsorbent with cost effectiveness and high potentiality. From the survey of about 80 -85 research papers, it was concluded that low cost adsorbents obtained from agricultural waste products were found to be having outstanding removal capabilities. This paper reviews the suitability of both raw and chemically modified agricultural products in the decolourisation of synthetic d yes.
Index Terms— Activated carbon, Agricultural waste, bio-adsorbents, chemically treated adsorbents , decolourisation, low-cost adsorbents, Synthetic Dyes.
—————————— ——————————
1 INTRODUCTION
Waste water from the textile industries mainly contain mod- erate concentration (10-200 mg.ml-1) of dyestuffs which con- tribute significant contamination of aquatic ecosystem (Neill et al, 1999). The demand of synthetic dyes has experienced the significant growth in the past decades and it was reported that more than 7×105 metric tons of various dyes are produced worldwide annually (Pearce et al,2003). Effluent discharged from dyeing industries is highly coloured and prove toxic to the aquatic ecosystem. Dyes are persistent in nature and strongly absorb sunlight which decreases the intensity of light absorbed by the water plants and phytoplankton, reducing photosynthesis and dissolve oxygen of the aquatic ecosystem and results in increase of COD. Further, dye effluents are highly dispersible, hard to treat, high in volume, hazardous in nature and made of harmful organic and inorganic chemicals that exhibit toxic and ca rcinogenic effects towards microbial population, human beings and animals (Reife et al, 1993).
———— ——— ——— ——— ———
Neetu Sharma, Assistant Professor in Environment Science, Bhagwan Mahavir Institute of Engineering & Tech nology, Sonipat,- 131001, Harya- na, India, PH-+91- 9813437047, E- Mail: Sharma.neetu80@gmail.com
Prof. D.P. Tiwari,Department of Chemical Eng ineering, DCRUST, Mur-
thal, Sonipat.India- 131039
Department of Civil Engineering, DTU, Delhi. India- 110042
During the last few decades, a no. of physical, chemical and biological methods were studied as coagulation, ultra - filtration, electrochemical adsorption, photo-oxidation and ion exchange method. Among them adsorption technique is gen- erally considered to be an effective method for quickly lower- ing the concentration of dissolved dyes in waste water (Reife et al, 1996). Activated carbon is the most widely used adsor- bent for dye molecules and dissolves compounds due to its high porosity and good surface area for sorption of organic compounds but high capital problem with handling of spent carbon, limits its widespread application. (Silva et al, 2006). In order to overcome this problem, the researchers are trying to develop low cost adsorbents as viable substitute for activated carbon. Over the years numerous low cost adsorbents were exploited as possible alternatives to activated carbon for the removal of hazardous chemicals. Some of the reported adsor- bents include clay material, siliceous materials, zeolites, agri- cultural wastes, industrial waste products, bio sorbents etc. (G.Crini, 2006).
In this article, the feasibility of various non -
conventional low cost adsorbents prepared from agricultural
waste has been reviewed. The main goal of this review is to provide a summary of recent information concerning the agri- cultural material as sorbents. Agricultural wastes are of low economic value, so inexpensive and abundantly available, mainly composed of cellulose, hemicelluloses and lignin which make them effective adsorbents for a wide range of pol- lutants due to the presence of functional groups such as h y- droxyl, carboxyl, methoxy, phenols, etc., that participates in binding with the pollutants (Hassanein & Koumanova, 2010).
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 2
ISS N 2229-5518
For this recently published papers were compiled. This review presents (a) variety of low cost adsorbents used in the recent past. (b) Describes the adsorption efficiency of treated and raw agricultural waste adsorbents (c) Adsorption isotherm models and thermodynamic parameters used to define the mechanism and process of adsorption.
SYNTHETIC D YES
A dye is a coloured substance that has an affinity to bind with the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fibers. Both dyes and pigments appear to be coloured because they absorb some light. In contrast with a dye, a pigment generally is insoluble, and has no affinity for the substrate. Synthetic dyes quickly replaced the traditional natural dyes. They are comparatively inexpensive and offered a wide range of new c olours, which imparted better properties to the dyed materials (Simon Ga r- field, 2000). Acid dyes are water-soluble anionic dyes that are applied to fibers such as silk, wool, nylon. Basic dyes are wa- ter-soluble cationic dyes that are mainly applied to acrylic fi- bers, but find some use for wool and silk. Direct dyes are used on cotton, paper, leather, wool, silk and nylon. Direct dyeing is normally carried out in a neutral or slightly alkaline dye bath, at or near boiling point, with the addition of either s o- dium chloride (NaCl) or sodium sulfate (Na2SO4 ). Mordant dyes require a mordant, which improves the fastness of the dye against water, light and perspiration. The choice of mor- dant is very important as different mordant can change the final colour significantly. Vat dyes are essentially insoluble in water and incapable of dyeing fibres directly. However, reduc- tion in alkaline liquor produces the water soluble alkali metal salt of the dye, which, in this leuco form, has an affinity for the textile fibre. Reactive dyes utilize a chromophores attached to a substituent that is capable of directly reacting with the fibre substrate. A chromophore is a radical configuration consisting of conjugated double bonds containing delocalised electrons such as azo (-N=N-), Carbonyl(=C=O),Carbon(=C=C=), Ca r- bon – Nitrogen (>C=NH or –CH=N-); nitoso (-NO or N-OH); nitro (-NO2 or =NO-OH); and sulphur (C=S) (S.J. Allen& B. Koumanova, 2005) The presence of ionising groups known as auxochromes results in maximum adsorption of the com- pound and provides a strong bonding affinity. Some common auxochromes groups include –NH3,-COOH, -HSO3, -OH (Al- Ghouti, 2004).
ADSOR PTION ISOTHERM MODELS
The process of adsorption is achieved by various pathways. Some adsorbents are held loosely by vander waals forces indi- cates the physical adsorption, while those held firmly indi- cates the chemical adsorption. The state of adsorbed molecule and the nature of bonding can be known by photoelectron spectroscopy. However the nature of adsorbed species can be very effectively studied by Infrared and Raman spectra under high pressure.The mechanism of adsorption can be studied by various adsorption isotherms. In order to understand the d e-
sign of adsorption process, various equilibrium curves have been described by the scientists.
Langmuir isotherm model
Langmuir proposed the monolayer adsorption of sorbet on homogenous adsorbent. The linear form of Langmuir (1918) isotherm model is represented by the Eqn.
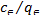 =
=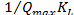 +
+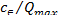 (1)
(1)
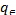
Where 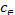 (mg/dm3) is the dye concentration in the solution at equilibrium, (mg/g) is the amount of dye adsorbed per unit mass of adsorbent. Qmax (mg/g) and KL (dm3 /g) are the Langmuir constants related to the theoretical maximum adsorption capacity and the energy of adsorption corresponds to a monolayer adsorption. The essential characteristics of Langmuir isotherm can be expressed by a dimensionless sepa- ration factor, RL. It indicates the nature of adsorption and ex- pressed as below:
(mg/dm3) is the dye concentration in the solution at equilibrium, (mg/g) is the amount of dye adsorbed per unit mass of adsorbent. Qmax (mg/g) and KL (dm3 /g) are the Langmuir constants related to the theoretical maximum adsorption capacity and the energy of adsorption corresponds to a monolayer adsorption. The essential characteristics of Langmuir isotherm can be expressed by a dimensionless sepa- ration factor, RL. It indicates the nature of adsorption and ex- pressed as below:
RL=1/1+KLC0 (2) Where C0 is the initial concentration (mg/dm3) and KL is
the Langmuir constant. Four possible conditions can be ob-
tained:
RL > 1 - Unfavourable, RL = 1 - Linear, RL = 0 - Irreversible,
0< RL < 1 - favourable
Freundlich isotherm model
Freundlich (1906) describe the equilibrium on heterogeneous surfaces and does not assume monolayer capacity and relates the adsorption with bulk. The linear form of Freundlich can be expressed by Eqn.
log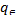 =logKF+1/nlog
=logKF+1/nlog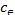 (3) Where KF is the Freundlich constant, representing the adsorp-
(3) Where KF is the Freundlich constant, representing the adsorp-
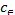
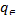
tion capacity (dm3/g) and n is the Freundlich exponent that
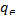
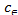
depicting the adsorption intensity (dimensionless). and stands for the same as described in Langmuir equation. The plot of versus log yields a straight line, with a slope of
1/n and intercept of lnKF . Greater will be the value of n more will be the adsorption.
Temkin isotherm model
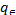
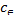
Temkin isotherm provides the relation between the amount adsorbed and the heat of adsorption. In chemisorptions, a d- sorption is accomplished by intake of energy or by release of energy showing the endothermic or exothermic processes. The linear form of Temkin isotherm model can be expressed by Eqn.
=BlnA+Bln (4)
Where A (dm3/gm) is the equilibrium binding constant and B
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 3
ISS N 2229-5518
is related to the heat of adsorption. Thermodynamic parameters
The values of thermodynamic parameters, confirms the nature
and type of adsorption. The various thermodynamic parame- ters such as Gibbs free energy (∆G), enthalpy (∆H), and entro- py (∆S) can be evaluated by following equations:
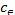 =Csolid/Cliquid (5)
=Csolid/Cliquid (5)
∆G=−RTln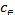 (6)
(6)
∆H=-R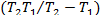 ln
ln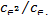 (7)
(7)
∆S = 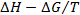 (8)
(8)
∆G= ∆H−T∆S (9) ln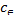 = −∆G/RT=∆S/R−∆H/RT (10) log
= −∆G/RT=∆S/R−∆H/RT (10) log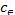 = ∆S/(2.303)R−∆H/(2.303)RT (11)
= ∆S/(2.303)R−∆H/(2.303)RT (11)
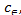
 are the equilibrium constants . Csolid and Cliquid
are the equilibrium constants . Csolid and Cliquid
(mg/l) are the concentration of solid and liquid phase respec- tively at equilibrium. T is the temperature in Kelvin and R is the gas constant. As the adsorption progresses the residual forces acting along the surface decreases. This in turn decreas- es the surface energy which appears as heat. This implies that adsorption accompanied by decrease in enthalpy of the sys- tem. The negative value of ∆G shows that adsorption is highly favourable and spontaneous. The positive value of ∆S shows increased disorder during the adsorption at the solid interface because of replacement of water molecule by dye molecule.
LO W COST ADSORBENTS USED FOR THE RE- MOVAL O F DYES
Low cost materials in their natural and modified forms have been extensively used as alternative adsorbents for dye re- moval (Ahmad et al, 2007; Gurses et al, 2006; Aksu and Tez- er,2000; Acemioglu,2004). According to Bailey et al, 1999, a sorbent can be considered as low cost if it require less processing, abundant in nature or is a by-product of some process and obtained as waste materials from another indus- tries. Clay material such as bentonite (Espantaleon et al, 2003), kaolinite (Ghosh and Bhattacharyya, 2002), montmorillonite, saponite (Alkan et al, 2004), diatomite (Al-Ghouti et al, 2003), and fuller’s earth (Atune et al, 2003), activated bleaching earth(W.T. Tsai et al 2004), were studied as low cost adsor- bents in their natural form for the removal of synthetic dyes. The adsorption capacities of clay results from a net negative charge present on the surface of minerals. This negative charge facilitates clay the capability to absorb positively
charge species. Their sorption properties also come from their highly porous structure. The use of zeolite and silicaceous ma- terial as silica beads, glasses, alunite, perlite and dolomite has also been observed for dye removal in recent past. Phan et al (2000) also showed that modified silica beads have better po- tential for the removal of acid dyes from coloured effluents. Use of modified alunite for the removal of acid dyes from waste water was conducted by özacar and Sengil, 2002. Other silicaceous material such as dolmite has also been proposed by Walker et al, 2003 for the removal of reactive dyes. The use of perlite as low cost adsorbent for the removal of dyes has been investigated for the first time by Alkan & co-workers, 2004. Several studies have also been conducted on the sorbent beh a- viours of natural zeolites for azo reactive dyes (Ozdemir et al,
2004; Armagan et al, 2004; Meshko et al 2001).
Industrial waste products have been also used extensively by
researchers because of its free or limited cost of availability.
The thermal power plant produce large amount of fly ash an- nually. The high percentage of silica and alumina in fly ash make it a good material for utilization. Fly ash has been used for the removal of phenol and chlorophenols (Haribabu et al,
1993), Heavy metals (pandey et al, 1985), dyes (Janos et al,
2003; Dizge et al, 2008; Kara et al, 2007; Tabrez et al,
2009).Various type of industrial waste such as blast furnace slag, dust and sludge obtained from steel industries has been investigated as adsorbents (Yamada et al, 1986 Dimitrova,
1996) for the removal of heavy metals. The adsorbents devel- oped from industrial waste have the tendency to remove inor- ganic contaminants (metal ions) more efficiently than organic constituents (dyes and phenols).
The carbon containing adsorben ts were made from biochemi-
cal and surplus sludge obtained from different plants by phys-
ical and chemical activation for the treatment of wastewater. The adsorption capacities of the sludge derived a dsorbents were observed better than the activated carbon by Lanlan Yu
& Qin Zhong, 2006.
Naturally occurring material with great abundance and their
low cost characteristics make them attractive adsorbents for
the dye removal. Among naturally occurring adsorbents chitin is abundant. It is found in exoskeleton of shell fish and crusta- cean animals and various researchers investigated it for dye removal (Yoshida et al, 1997; Chiou et al, 2002; Chiou et al,
2004). Peat is another naturally occurring material containing
lignin and cellulose. Maria investigated adsorption characte-
ristics of peat along with fly ash and bentonite and found that it was having maximum adsorption capacities than the rest two. Wood( Poots et al, 1976), natural coal (Mittal et al, 1993) water hyacinth (Varghese et al, 2004) were also studied by the researchers as low cost adsorbents for the decolourisation of dyes.
AGRICULTUR AL WA STE USED IN THE DEC O- LOURISATION O F SYNTHE TIC D YE S
Large amount of agricultural waste is produced by most of the countries every year and major part of this waste is utilized as domestic fuel. The abundance and widespread availability of agricultural by-products make them good source of raw mate-
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 4
ISS N 2229-5518
rials for dye removal. Several agricultural waste products like rice husks, corncob, coir-pith, plum kernels, bagasse pith, nut shells, fruit peels, leaf powders, used tea leaves, fruit shells, seed husk, saw dust, hyacinth root etc were tried as alternative low cost adsorbent by various researchers in recent past dec- ades.
ACTIVATED C ARBON PREPARED FROM AGRI- CULTUR AL WASTE
Plentiful agricultural waste and various unused plant parts offer an inexpensive and renewable additional source of acti- vated carbon (AC). These waste materials have little or no economic value and often pose a great disposal problem. So these waste materials are used in treated and untreated form for the removal of dyes. Porter demonstra ted that adsorption of AC is an effective and complete treatment for the textile waste water. Preparation of ACs from a wide range of agro waste for treatment of waste water has been reported earlier by Pollard et al, 1992. A wide variety of activated carbon pre- pared from agro- waste such as pine- wood (Tseng et al, 2003), Corn cob (Robinson et al, 2002), fruit stones and nut shells (Aygün et al, 2003 and Sumanjit et al, 2007), Cassava peel (Ra- jeshwarisivraj et al, 2001), Tapioca peel ( Parvathi et al, 2010) Bamboo(Wu et al, 1999), bagasse (Tsai et al, 2001 and Ragh u- vanshi et al, 2004), rice husk (Jyoti Sharma and Beena Janvi,
2008; Chandrasekhar and Pramada, 2006; Y.C. Sharma et al
2009), bark (Shanmugam Arivoli et al, 2009; L.S. Tan et al,
2010), leaves (Hema & Arivoli , 2008 a nd M.C. Nci bi et al .,2007) a nd
Used tea leaves( Sumanjit et al., 2007).
Kaushik et al 2004 demonstrated that dye removal is more effective with chemically activated bagasse in comparison to raw bagasse. The activated rice husk carbon prepared simply by steam was proved as a favourable low cost adsorbent for the removal of congo red dye was investigated by Jyoti Sha r- ma and Beena Janvi, 2008. It was observed that an amount of
0.08g/l of RHCAS could remove 10 -99% of dye from an
aqueous solution of 25 ppm. Application of activated carbon prepared from rice husk, was estimated as a potential adsor- bent for the removal of Malachite green and found to be ha v- ing good adsorption capacity comparative to the activated carbon prepared from banana peel (Annadurai et al,2002), date pits(Banat et al, 2003), rice husk (Kannan and Sundaram et al, 2001), wood saw dust (Namasivayam et al 2001), orange peel (Annadurai et al,2002), sugarcane dust (Khattri and Singh et al, 2000), activated carbon (Sharma et al,2007) except the coconut husk(Sharma et al,2009). Kaushik et al (2004) reported that adsorption on activated bagasse increases from 78.09% to
86.35% with rise in temperature from 30-50 ċ with 4 gm/l dose
from 100 ppm of dye solution. Garg et al (2003) demonstrated that adsorption efficiency of sulphuric acid treated saw dust was higher than formaldehyde treated saw dust for the re- moval of malachite green. It was concluded tha t ACR adsorp- tion efficiency was unaffected by pH, while 6 -9 pH was opti- mum for dye removal by SDC and SD. The adsorption of ash prepared from rice husk was found to be an effective adsor- bent because of their high surface area and the volume. The
maximum monolayer capacity estimated was 690mg/g. Moreover the adsorption was maximum on the ash compared to the activated carbon prepared from the rice husk after calc i- nations because of the presence of both silica and carbon (Chandrasekhar and Pramada, 2006).
The adsorption characteristics of direct red-23 on to mangrove bark (Rhizopus apiculata) that has been previously treated with formaldehyde in acid medium was investigated and ob- served that dye sorption decreases at high pH values in accor- dance with the ion exchange mechanism of the adsorption and maximum removal was at 2. The monolayer sorption capacity of modified bark for DR-3 sorption was found to be 21.55 mg/g. (Tan et al 2010) A carbonaceous adsorbent prepared from banana peel was investigated as considerable adsorbent in the removal of rhodamine B with the adsorption capacity of
40.161 mg/g at 30ċ and 7 pH by Shanmugam et al, 2009. The
positive value of ∆H indicates the physiosorption and the en- dothermic nature of adsorption. The adsorption of Malachite green on the carbonaceous material prepared by dried leaves of pandanus was observed by Hema et al, 2008. The maximum adsorption capacity was found to be 9.73 mg/g at 6 pH and
30ċ. Activated carbon prepared by impregnation of H 3PO4 fol- lowed by activation at 800ċ of Euphorbia antiquorum L wood was used as adsorbent by Sivakumar and Palanisamy, 2010, for the removal of Acid Blue 92, Basic Red 29, Reactive Red 4 and Direct Blue 53. It was demonstrated that the selected a d- sorbent was mesoporous and can accommodate multilayer dye adsorption due to its high pore width and pore diffusion plays a significant role in the adsorption than film diffusion. The removal efficiency of activated carbon prepared from agricultural adsorbents depends on various factors such as surface area, nature of charge present, pore structure, chemical composition and mechanism of adsorption. The removal effi- ciency also depends upon the characteristics of sorbet which is to be adsorbed. The characteristics of sorbet vary with signifi- cant variation in concentration, contact time and pH. Adsorp- tion is a complicated process and not accomplished by a sp e- cific mechanism. So much work is still required to identify the mechanism of adsorption.
RAW AGRICULTURAL WAST E USED A S AD- SORB ENTS
Literature from past decades reports several studies on the effective adsorption of dyes by raw adsorbents prepared from agricultural waste and unused plant parts. Several agricultural solid wastes from cheap and readily available sources were tried in the natural form in the recent past for the removal of dyes to avoid the cost of chemicals and complicated processes of modification. Such as tamarind fruit shell (M.C Somasekha- ra Reddy., 2006), Jack fruit peel, a nanoporous a dsorbent ( M. Jayarajan et al., 2011), gra pefruit peel (Abassi & Razzaghi.,
2009), rice husk (Ola Abdelwahab et al., 2005), yellow passion
fruit peel (Flavio Andre Pavan et al., 2008) have also been su c- cessfully employed in raw form for the removal of dyes from aqueous solution.
Bark is an abundant forest residue which has been found to be effective in removing dyes from aqueous solution (McKay
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 5
ISS N 2229-5518
et al., 1999). Bark is an effective adsorbent because of its tannin content (Bailey et al., 1999; Morais et al., 1999). Teak tree bark powder was used as a ttractive adsorbent for the adsorption of methylene blue by Satish Patil et al., 2011. The uptake by raw TTBP adsorbent was found to be 333.3 mg/g and increased with increasing pH.
The adsorption of congo red, a direct azo dye on hazel-
nut shell was estimated by Riccardo A. Carletto et al., 2008.
Hazlenut shells were employed as organic support for the cu l- ture of phanerochaete chrysosporium to take macronutrients as carbon and nitrogen from hazelnut shells and biodegrada- tion of adsorbed dyes was s tudied. The maximum amount of congo red adsorbed on hazelnut shell was 13.75 mg/g. Saw dust is an abundant by-product of the industry that is either used as cooking fuel and packing material. The role of saw dust materials in the removal of pollutants from aqueous solu- tions has been reviewed recently (Shukla et al., 2002). Chemi- cal pre-treatment of sawdust has been shown to improve the sorption capacity and enhance the efficiency of saw dust a d- sorption (Garg et al., 2003, 2004a,; Batzias and Sidiras, 2004). Batch adsorption of methylene blue and acid blue onto ground nut hazelnut shell was studied in comparison with saw dust of various species of wood by F. Ferrero, 2006. The author ob- served the higher adsorption capacity of hazelnut shell than wood sawdust obtained from various species of wood, to- wards both dyes.
The sorption characteristics of sunflower seed husk was investigated by Siew-Teng Ong et al, 2010, to remove meth y- lene blue under batch condition and maximum sorption c a- pacity was found to be 45.25 mg/g also the sorption was found to be pH, concentration and agitation dependent. The granular bio adsorbent prepared from the fruit peel of Cuc u- mis sativa could effectively remove methylene blue, methyl red and malachite green from aqueous solution at the pH 6 and the maximum adsorption capacity was 140.84 mg/g for methyl red (T.Santhi et al., 2009).
Naturally available agricultural waste, wheat straw
was evaluated for the treatment of basic yellow 21 with max i-
mum adsorption capacity of 71.43 mg/g by Taha F. Hassanein
& B. Koumanova, 2010. The adsorption mechanism was su g-
gested to be complex, consisting of both surface adsorption
and pore diffusion. A nanoporous adsorbent prepared from jackfruit peel waste was found to be an attrac tive adsorbent for the removal of rhodamine B at low pH of 4.3 with max i- mum removal efficiency of 4.361 to 1.98 mg/g (M.Jayarajan et al, 2011). The rate of adsorption increases with increase in temperature indicating that the sorption is an endothermic process and rising temperature enhance the mobility of dye which facilitates the adsorption process. Increase in tempera- ture enhance the sorptive interaction between the active sites of sorbent and sorbate ions. It is also said that increasing tem- perature may also produce a swelling effect in the internal structure of the carbons enhancing more dye adsorption on the sorbents (S. Senthilkumaar et al., 2006). The rate of adsorp- tion of malachite green on neem bark powder and mango bark powder increases with increase in temperature was demon- strated by Srivastva & Rupainwar, 2010. Also mango bark powder was suggested better adsorbent than neem bark
powder.
The sorption efficiencies of corncob and ba rley husk with
different particle size and weight for various dyes were dem- onstrated by T.Robinson et al. It was observed by the author that 2gm of barley husk and corn cob was more efficient than
5gm resulted in 92% of dye removal while the paricle size of
≤600 µm, for both substrates, had the higher rate of remova l
than 1×4mm particle size in the first five hours, and further
removal occurred at much slower rate. It may be expected because smaller particle have large specific surface
CONCLUSION
This review presents the efficiency of low cost agricultural adsorbents for dye removal. The comparison between the raw and treated adsorbents is summarised briefly. From the recent literature reviewed it is demonstrated that chemically treated agricultural waste showed comparatively significant removal efficiency than the raw agricultural waste. Decolourisation process is not specific and depends upon many factors. Al- though there are lots of agricultural adsorbents which can act as a substitute for the expensive commercial activated carbon but complete replacement is not possible. The factors which favour the selection of agricultural a dsorbents are its low cost, widespread presence and organic composition which shows strong affinity for some selected dyes.
ACKNOWLEDGM ENT
The author acknowledges sincere thanks to B.M. In stitute of Engineering and Technology, Sonepat, Haryana, India for ca r- rying out the research work successfully and for providing the assistance for collection of material for the review.
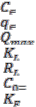
Nomenclature
= concentration of dye at equilibrium
= amount of dye adsorbed
= maximum adsorption capacity
= energy of adsorption
= Separation factor
Initial dye concentration
= Freundlich constant related to adsorption capacity
n= Freundlich exponent depicting the adsorption capacity
∆G= Gibbs free energy
∆S= Entropy change
∆H= Enthalpy
R= Gas Constant
T= Temperature in Kelvin RHCAS= Rice husk carbon SDC= Saw dust carbon SD= Saw dust
ACR= activated carbon
DR= Direct red
TTBP= Teak tree bark powder
A= Equilibrium binding constant
B= Heat of adsorption
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 6
ISS N 2229-5518
TABLE 1
ADSORPTION CAPACITIES OF TREATED AGRICULTURAL WASTE
Dyes Activation Process
Equillibrium
time qɛ(mg/g) References
Rhodamine B Ca rbonize d by Conc. 40 min 40.161 Shanmuga m Arivoli et al(2009) H2SO4 & activated by
heating at 600ċ
Direct F. Sca rlet a ctiva ted by 0.6M 90 min 4.35 Ola Abdelwahab et al(2005)
citric acid for 20ċ
Direct red-23 Chemica lly treated 4 hrs 21.55 L.S. Tan et al(2010)
with a mixture of 37% HCHO+0.2N H2SO4
Ma lchite Green Ca rbonized by Conc. 40 min 9.737 Hema&Arivoli(2008) H2SO4 & activated by
heating at 400ċ for 12hrs
Reactive red 23 Chemica lly treated with 10 hrs 0.31 M.C. Ncibi et a l(2007)
a mixture of 0.2 HNO3
Methylene blue As h is formed by hea ting 30 min 690 Cha ndras ekha r&Pra mada (2006)
a t 500ċ for 2 hrs
Red Brown C4R Activa ted by conc. H2SO4 45 min 121.47 C. Pa rvathi et a ( 2010)
a nd ca rbonized a t 200ċ
Acid violet 17 Cha rcoa l prepa red by 4 hrs 38.32 Suma njit et a l (2007)
burning in open a ir
Acid violet 17 do 100.57 Suma njit et a l (2007) Acid Red 119 do 4 hrs 44.48 Suma njit et a l (2007) Acid Blue15 do 4 hrs 126.53 Suma njit et a l (2007)
Acid Red 119 do 72.81 Suma njit et a l (2007)
Ma lachite green Ca rbonized in quartz tube 40 min 63.85 Y.C.Sha rma et a l(2009)
in muffle furna ce at 450ċ
for 1 hrs & a ctiva ted a t
650ċ for 2 hrs .
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 7
ISS N 2229-5518
TABLE 2
ADSORPTION CAPACITIES OF RAW AGRICULTURAL WASTE
Adsorbents | Dye | equilibrium time | Temperature(ċ) | qɛ(mg/g) | References |
Neem Ba rk | Ma lchite Green | 7 hrs | 25ċ | 0.36 | Srivasta va&Rupa inwa r(2010) |
Ma ngo Bark | Ma lchite Green | 7 hrs | 25ċ | 0.53 | Srivasta va&Rupa inwa r(2010) |
Ta ma rind s hell | Congo red | 4 hrs | 30±1ċ | 10.48 | Somas ekha ra Reddy(2006) |
Neem lea f powder | Congo red | 5 hrs | 27ċ | 72 | Bhattacha rya&Arunima (2004) |
Gra pe fruit peel | Reactive blue 19 | 45 min | 25ċ | 12.53 | Abass i& Ra zza ghi As l(2009) |
Teak tree ba rk | Methylene blue | 30 min | 30ċ | 333.3 | Pa til et al(2011) |
Whea t s tra w | Basic Yellow 21 | 48 hrs | 20±2ċ | 71.43 | Hassa nein&Koumanova (2010) |
s unflower seed husk | Methylene blue | 4 hrs | 25±2ċ | 45.25 | Siew-Teng Ong et a l(2010) |
Ha zlenut s hell | Methylene blue | 60 min | 20ċ | 76.9 | F.Ferrero(2007) |
Ha zlenut s hell | Acid Blue | 60 min | 20ċ | 60.2 | F.Ferrero(2007) |
Cherry saw dust | Methylene blue | 2 hrs | 20ċ | 39 | F.Ferrero(2007) |
Wa lnut sa w dus t | Methylene blue | 60 min | 20ċ | 59.17 | F.Ferrero(2007) |
Oa k sa w dust | Acid Blue | 60 min | 20ċ | 29.5 | F.Ferrero(2007) |
Pitch Pine sa w dust | Acid Blue | 60 min | 20ċ | 27.5 | F.Ferrero(2007) |
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 8
ISS N 2229-5518
REFERENCES
[1] Aygün, S. Yenis oy-Karakas ., I. Duman, ―Production of gra- nular activated carbon from fruit stones and nut shells and evaluation of their physical, chemical and adsorption properties ,‖ Micropor. Mesopor. Mater, 66, 189-195, (2003).
[2] Gurs es , C. Dogar, M. Yalcin, M. Acikyidiz, R. Bayrak, S.
Karaca,― The ads orption kinetics of cationic dye methylene
blue onto clay,‖ J.of Hazard. Mater, 131, 217-228, (2006). [3] Khan Tabrez, Imran Ali, Ved vati Singh and Sangeeta
Sharma, ―Utilization of flyas h as low cost adsorbent for the
removal of methyene blue, Malchite Green and rhodamine
B dyes from textile was te water.‖ J. of Environ. Poll. Sci.,
3, 11-22, (2009).
[4] Reife, in: J. I. Kroschwitz, M. Howe-Grant(Eds.), Kirk- Othmer Encyclopedia of Chemical Technology, Vol. 8, fourth ed., Wiley, New York, 1993.
[5] Reife, H.S. Freeman, in: A. Reife, H.S. Freedom (Eds.),
Environmental chemistry of dyes and pigments, Wi- ley, Newyork, 1996.
[6] Shukla, Y.H. Zhang, P. Dubey, J.L. Margrave, S.S. Shukla,― The role of s aw dus t in the removal of unwanted materials from water,‖J. Hardous Mater, B95, 137-152, (2002).
[7] A.A Ahmad, B.H. Hameed, N. Aziz, ―Adsorption of direct
dyes on palm as h: Kinetic and equilibrium mo delling,‖ J. of
Hazard. Mater, 141, 70-76, (2007).
[8] A.Carletto Riccardo, Fabiana Chimirri, Frans esca Bos co and Franco Ferro, ―Adsorption of congo red dye on hazel- nut shell and degradation with Phanerochaete chryosos po- rium,‖ Bio Res . 3(4), 1146-1155, (2008).
[9] A.G. Espantaleon, J.A. Nieto, M. Fernandez, A. Ma r- sal,― Use of activated clays in the removal of dyes and surfactants from waste water,‖ Appl. Clay Sci., 24,
105-110, (2003).
[10] A.K. Mittal, C. Venkobachar, ―Sorption and des orption of
dyes by sulfonated coal,‖ J. Environ. Engg. ASCE, 119,
366,-368, (1993).
[11] Andre Pavan Flavio, Ana Cris tina Mazzocato, Yos hitaka Gus hikem,― Removal of methylene blue dye from aqueous solution by ads orption us ing yellow pass ion fruit peel as adsorbent,‖ Biores ource Technology, 99, 3162-3165, (2008).
[12] Arivoli Shanmugam, M. Thenkukuzhali and P. Martin Deva Prasath, ―Adsorption of rhodamine B by acid activated car- bon: Kinetic, Thermodynamic and Equilibrium Studies ,‖ The Electronic Journal of Chemis try. 1(2), 138-155, (2009).
[13] Acemioglu, ―Ads orption of congo red from aqueous solu-
tion onto calcium rich fly as h,‖ J. Colloid Interface Sci.,
274, 371-379, (2004).
[14] Armagon, M. Turan, M.S. Celik, ―Equilibrium studies for the adsorption of reactive azo dyes onto zeolite,‖ Desalination, 170, 33-39, (2004).
[15] Namas ivayam, D.M. Kumar, K. Selvi, ―Was te coir pith – a
potential biomass for the treatment of dyeing was te wa- ters ,‖ Biomass Bioenergy, 21, 477-483, (2001).
[16] Parvathi, T. Maruthvanan, S. Sivamani, C. Prakash and
C.V. Kous hik, ―Role of tapioca peel activated carbon
(TPAC) in decolouris ation of Red Brown C4R reactive
IJSER © 2012
dye,‖ Ind. J. of Sci. and Tech., 3(3), 290-292, (2010).
[17] C.L. Pearce, J.R. Lloyd, J.T.Guthrie,― The removal of colour from textile waste water using whole bacterial cells: A review,‖ Dyes Pigment, 58(3), 179-196, (2003)
[18] C.O’. Neill, F.R. Hawkes, D.L. Hawkes, N. Lourenco,
H.M. Pinheiro, W. Delee, ―colour in the textile efflu- ents- sources, measurements discharge consents and simulation: a review,‖ J. Chem. Technol. Biot.,74(11),
1009-1018, (1999).
[19] Ghosh, K.G. Bhattacharyya, ―Adsorption of meth ylene blue
on kaolinite,‖ Applied Clay Science, 20, 295-300, (2002).
[20] Haribabu, Y.D. Upadhya, S.N. Upadhyay, ―Removal of phenols from effluent by flyash,‖ Int. J. Enviro. studies, 43, 169-176, (1993).
[21] Banat, S., Al- Asheh, L., Al-Makhadmeh,―Evaluation of the us e of raw and activated date pits as potential adsorbents for dye containing water, ‖ Process Biochem., 39, 193-202, (2003).
[22] Ferrero,― Dye removal by low cost adsorbents : Hazelnut
shell in comparison with wood s aw dus t,‖ Journal of Ha- zardous Materials , 142, 144-152, (2007).
[23] Hassanein Taha, and B. Koumanova, ―Evaluation o f ad-
sorption potential of the agricultural was te wheat straw for Bas ic Yellow 21,‖ Journal of the univers ity of Chemical Technology and Metallurgy, 45(4), 407-414, (2010)
[24] F.C.Wu, R.L. Ts eng, R.S. Juang, ―Preparation of activated
carbons from bamboo and their adsorption ability for dyes and phenol,‖ J. Environ. Sci. Health A., 34, 1753-1775, (1999).
[25] Annadurai, R.S. Juang, D.J. Lee, ―Us e of cellulos e-bas ed wastes for adsorption of dyes from aqueous solution,‖ J. Hazard Mater, B92, 263-274, (2002).
[26] G. Atun, G. Hisarli, W.S. Sheldrick, M. Muhler, ―Ad- sorptive removal of methylene blue from coloured effluents on fuller’s earth,‖ J. Colloid Int. Sci. 261, 32-
39, (2003).
[27] G. McKay, J.F. Porter, G.R. Pras ad, ―The removal of dye
colours from aqueous solutions by adsorption on low cost
materials ,‖ Water Air Soil Pollut. 114, 423-438, (1999).
[28] G.Crini,― Non-Conventional low cost adsorbents for dye removal: A review,‖ Bioresource technology, 97,
1061-1085, (2006).
[29] G.M. Walker, L. Hansen, J.A. Hanna, S.J. Allen, ―Ki-
netics of a reactive dye adsorption onto dolomite sor-
bents,‖ Water Res., 37, 2081-2089, (2003).
[30] Yamada, M. Kayama, K, Saito and K.M. Hara, ―A fund a-
mental res earch on phos phate removal by us ing s lag,‖ Wa- ter Res ., 20(5), 547-577, (1986).
[31] H. Yoshida and Takemori, T., ―Ads orption of direct dye on
cross -linked chitos an fiber,‖ Water Sci. Technol, 35, 29-
37, (1997).
[32] Silva, A. Pereira, Barata, Fonseca, J. Faria, ―Adsorp- tion of reactive dye on chemically modified activated carbons – influence of pH,‖ J. Colloid interface Science, 296(2), 480-489, (2006).
[33] Jyoti Sharma and Beena Janveja― A study on removal of
congo red dye from the effluent of textile indus try us ing rice hus k carbon activated by s team,‖ Rasayan J. Chem.,4,
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 9
ISS N 2229-5518
936-942,(2008).
[34] K.G. Bhattacharyya, Arunima Sharma,― Azadirachta indica leaf powder as an effective biosorbent for dyes : a case study with aqueous Congo Red solutions ,‖ J. Environ. Manage., 71, 217-229, (2004).
[35] K.K. Pandey, G. Prasad, V.N. Singh, ―Copper(II) re- moval from aqueous solutions by fly ash ,‖ Water Res., 19, 869-873, (1985).
[36] K.S. Low, C.K. Lee, ― The removal of cationic dyes us ing coconut hus k as an adsorbent,‖ Pertanika, 13(2), 221-228, (1990).
[37] L.C. Morais , O.M, Freitas , E.P. Goncalves , L.T. Vas conc e- los , C.G. Gonzalez Beca, ―Reactive dye re moval from waste waters by adsorption on eucalyptus bark: variables that define the process ,‖ Water Res . 33, 979-988, (1999).
[38] L.S. Tan, K. Jain, C.A. Rozaini, ―Adsorption of textile dye
from aqueous solution on pretreated mangrove bark, an agricultural waste: Equilibrium and Kinetics s tudies ,‖ Jou r- nal of Applied Sciences in Environmental Sanitation, 5(3),
283-294, (2010).
[39] M. Jayarajan, R. Arunachalam and G.Annadura, ― Agricu l- tural waste of jack fruit peel nano -porous adsorbent for the removal of rhodamine dye,‖ As ian Journal of Applied Sciences , 4(3), 263-270, (2011).
[40] M. Alkan, ö. Demirbas , S. Celikcapa, M. Dogan, ―Sorption
of acid red 57 from aqueous solution onto s epiolite,‖ J. of
Hazardous Mater, 116, 135-145, (2004).
[41] M. Alkan, ö., Demirbas, S. Celikcapa , M., Dogan, D.D. Adrian, ―A review of potentially low-cost sor- bents for heavy metals,‖ Water Res., 33, 2469-2479, (1999).
[42] M. Hema, S. Arivoli, ―Adsorption Kinetic and thermody-
namics of malachite green dye into acid act ivated low cost carbon,‖ J. App. Sci. Environ. Manage., 12(1), 43-51, (2008).
[43] M. Özacar, A.I. Sengil, ―Adsorption of metal complex dyes from aqueous solutions by calcined alunite and granular activated carbon,‖Adsorption, 8, 301-308, (2002).
[44] M.A. Al-Ghouti, M.A.M. Khraisheh, S.J. Allen, M.N
Ahmad, ―The removal of dyes from textile waste wa-
ter; a study of the physical characteristics and adsorp- tion mechanism of diatomaceous earth,‖ J. Environ. Manage., 69, 229-238, (2003).
[45] M.C. Ncibi, B. Mahjoub, M. Seffen,― Adsorptive removal of textile reactive dye us ing Pos idonia oceanic (L.) fibrous biomass ,‖Int. J. Environ. Sci. Tech, 4(4), 433-440, (2007).
[46] M.C. Somasekhara Reddy, ―Removal of direct dye from aqueous s olution with an adsorbent made from tamarind fruit shell, an agricultural s olid was te,‖ Journal of Scientific and Industrial Res earch, 65, 443-446, (2006).
[47] M.S. Chiou, Ho PY and Li HY, ―Adsorption of anionic
dyes in acid solutions us ing chemically cross -linked chito-
s an beads .‖ Dyes Pigments , 60, 69-84, (2004).
[48] M.S. Chiou, Ho, P.Y. and Li, H.Y., ―Equilibrium and kinetic modelling of ads orption of reactive dye on cross -linked chi- tosan beads‖ J. Hazard Mater, 93, 233-248, (2002).
[49] Mahmood Abass i and Nima Razzaghi As l, ―Removal of
hazardous reactive blue 19 dye from aqueous solution by agricultural was te,‖ J. Iran. Chem. Res .2, 221-230, (2009).
[50] N. Dizge, C. Aydiner, E. Demirbas, M. Kobya, S. Kara,
―Adsorption of reactive dyes from aqueous solution
by flyash: Kinetic and equilibrium studies,‖ J. Hazard.
Mater, 150, 737-746, (2008)
[51] N. Kannan, M.M. Sundaram,― Kinetics and mechanis m of
removal methylene blue by adsorption on various carbons -
a comparative study, ‖ Dyes Pigments , 51, 25-40, (2001).
[52] O. Ozdemir, B. Armagon, M. Turan, M.S. Celik,― Comparision of the adsorption characteristics of azo reactive dyes on mezoporous minerals,‖ Dyes Pig- ments, 62, 49-60, (2004).
[53] Ola Abdelwahab, Ahmed EL Nemr, Amany EL Sikaily, A z-
za Khaled, ―Use of Rice hus k for adsorption of direct dyes from aqueous s olution: A cas e study of Direct F. Scarlet,‖ Egyptian Journal of Aquatic res earch, 31(1), 1110-0354, (2005).
[54] P. Janos, H. Buchtova, M. Ryznarova, ―Sorption of
dyes from aqueous solutions onto flyash,‖ Water Res.,
37, 4938-4944, (2003).
[55] R. Srivastava and D.C Rupainwar, ―A comparative evalu a-
tion for ads orption of dye on neem bark and mango bark
powder,‖ Indian Journal of Chemical Technology, 18, 67-
75, (2011).
[56] R.L. Ts eng, F.C. Wu, R.S. Juang, ―Liquid phas e adsorption of dyes and phenols us ing pine-wood based activated car- bons ,‖ Carbon, 41, 487-495, (2003).
[57] Rajeshwaris ivaraj, S. Sivakumar, P. Senthilkumar, V. Su b-
buram, ―Carbon from cassava peel, an agricultural waste, as an adsorbent in the removal of dyes and metal ions from aqueous s olution,‖ Bioresource Technology, 80, 233-235, (2001)
[58] S. Chandras ekhar, P.N. Pra mada, ―Rice hus k as h as an ad-
sorbent for methylene blue- effect of ashing temperature,‖
Ads orption, 12, 27-43, (2006).
[59] S. Kara, C. Aydiner, E. Demirbas, M. Kobya, N.Y.
Dizge, ―Modelling the effects of adsorbent dose and
particle size on the adsorption of reactive textile dye by flyash.‖ Desalination, 212, 282-293, (2007).
[60] S. Varghese, V.P. Vinod and T.S., Anirudhan, ―Kinetic and equilibrium characterization of phenols adsorption onto a novel activated carbon in water treatment,‖ Indian J. Chem. Technol., 11, 825, (2004).
[61] S.D. Khattri, M.K. Singh, ―Colour removal from s ynthetic
dye waste water us ing a bio sorbent,‖Water Air Soil Pollu.,
120, 283-294,(2000).
[62] S.E. Bailey, T.J. Olin, M. Bricka, D.D. Adrian,―A review of potentially low cost adsorbents for heavy metals ,‖ Water Res ., 33, 2469-2479, (1999).
[63] S.F. Bailey, T.J. Olin, M. Bricka, D.D. Adrian,―A review of potentially low cos t ads orbent for heavy metals . ‖ Water Res .33, 2469-2479,(1999).
[64] S.J.T. Pollard, G.D. Fowler, C.J. Sollars , R. Perry, ―Low cost adsorbents for was te and was te water treatment: a re- view,‖ The Sci. Total Environ. 116, 31-52, (1992).
[65] S.P. Raghuvanshi, R. Singh and C.P. Kaus hik,― kinetics study of methylene blue dye Bioadsorption on bagasse,‖
IJSER © 2012
http :// www.ijser.org
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 3, Issue 2, February -2012 10
ISS N 2229-5518
Applied Ecology and Environmental Research . 2(2), 35-43, (2004).
[66] S.V Dimitrova,―Metal sorption on blast furnace s lag,‖ Wa- ter Res ., 30, 228-232, (1996).
[67] Satish Patil, Sameer Renukdas , Nas eema Patil ―Removal of methylene blue, a bas ic dye from aqueous s olution by a d- sorption us ing teak tree(Tectona g randis ) bark powder,‖ Int. J. of Env. Sci., 1(5), 711-725, (2011).
[68] Siew-Teng Ong, Pei-s in Keng, Siew-Ling Lee, Ming-How Leong and Yung-Ts e Hung, ―Equilibrium s tudies for the removal of bas ic dye by s unflower seed hus k (Helianthus annus ),‖ International Journal of Phys ical Sciences , 5(8),
1270-1276, (2010).
[69] Simon Garfield, Mauve: How One Man Invented a Color
That Changed the world, (2000).
[70] Siva Kumar Ponnus amy and Nachimuthu Palanis amy,
―Mechanistic s tudy of dye ads orption onto a novel non - conventional low cos t adsorbent,‖ Advances in Applied Science res earch, 1(1), 58-65, (2010).
[71] Sumanjit, T.P.S. Walia, Ravneet Kaur, ―Removal of health
hazards caus ing acidic dyes from aqueous solutions by the process of adsorption,‖ Ojhas , 6 (3), 1-10, (2007).
[72] T. Robinson, B. Chandran, P. Nigam, ― Removal of dyes
from an artificial textile dye effluent by two agricultural waste res idues , corncob and barley hus k,‖ Environment In- ternational, 28, 29-33, (2002).
[73] T. Robins on, B. Chandran, P. Nigam., ―Re moval of dyes from an artificial textile dye effluent by two agricultural waste res idues , corn cob and barley hus k,‖ Environ. Int.,
28, 29-33, (2002).
[74] T. Santhi, S. Manonmani, T. Smitha, D. Sugritha and K.
Mahalaxmi, ― Uptake of cationic dyes from aqueous solu- tion by bio-ads orption onto granular Cucumis sativa,‖ Journal of Applied Sciences in Environmental Sanitation,
4(1), 29-35, (2009).
[75] T.N.T Phan, M. Baequet, M.Morcellet, ―Synthesis and characterization of silica gel functionalized with mo- nochlorotriazinyl beta-cyclodextrin and their sorption capacities towards organic compounds,‖ J. Inclusion Phenom. Macrocyclic Chem., 38, 345-359, (2000).
[76] V. Meshko, L. Markovska, M. Mincheva, A.E. Rodri-
gues, ―Adsorption of basic dyes on granular activated carbon and natural zeolite,‖ Water Res., 35, 3357-3366, (2001).
[77] V.J.P. Poots , G. McKay, J.J. Healy, ―The removal of acid dye from effluent us ing natural adsorbents -I. Peat.‖ Water Res ., 10, 1061- 1067, (1976).
[78] V.K. Garg, R. Gupta, A.B. Yadav and R. Kumar, ― Dye re- moval from aqueous solution by adsorption on treated s aw dust,‖ 89, 121-124, (2003).
[79] W.T. Tsai, C.Y Chang, C.H. Ing and C.F. chang, ―Adsorp- tion of acid dyes from aqueous solution on activated bleaching earth.‖ J. of Coll. and Inter. Sci., 275, 72-
78,(2004).
[80] W.T. Tsai, C.Y. Chang, M.C. Lin, S.F. Chien, H.F. Sun, M.F. Hs ieh, ―Adsorption of acid dye onto activated carbon prepared from agricultural was te bagass e by ZnCl2 activa- tion,‖ Chemos phere, 45, 51-58, (2001).
[81] Y.C. Sharma, B. Singh and Uma, ― Fas t removal of ma l- chite green by adsorption on rice hus k activated carbon,‖ The Open Environmental Pollution and To xicology Journal,
1, 74-78, (2009).
[82] Y.C. Sharma, Uma, V. Srivastava, J. Srivas tava, M. Mahato,
― Reclamation of Cr(VI) rich water and was tewater by wollas tonit, ‖Chem.Eng.J., 127, 151-156,(2007).
[83] Yu Lanlan, Q. Zhong,― Preparation of adsorbents made from s ewage s ludges for adsorption of organic materials from was tewater,‖J. Jhazmat, B137, 359-366, (2006).
[84] Z. Aksu, S. Tezer, ―Equilibriu m and kinetic modelling of
biosorption of Remazol Black B by Rhizopus arrhizus in a
batch sys tem: Effect of temperature.‖ Proc. Biochem., 36,
431-439, (2000).
IJSER © 2012
http :// www.ijser.org