The research paper published by IJSER journal is about Current measurement using Ferroelectrics like Lead Bismuth Niobate and PZT 1
ISSN 2229-5518
Current measurement using Ferroelectrics like
Lead Bismuth Niobate and PZT
Parnashi Chakraborty
Abstract— This paper states the application of a ferroelectric substances in measurement of current using the property that their dielectric constant increases with temperature. Since the change in dielectric constant with temperature is very steep, the resolution of the current measuring device will be very high. Binary and ternary oxides have been used based on perovskite systems. The compounds have been synthesised, characterised with respect to their crystal structure and their dielectric constants have been measured
Index Terms— Ferroelectric substances, Current measurement, PZT, Lead Bismuth Niobate, Dielectric strength, Temperature, perovsk ites
—————————— ——————————
1. FERROELECTRIC MATERIAL
To be ferroelectric, a material must possess a spontaneous dipole moment that can be generated by an applied electric field, i.e. spontaneous switchable polarisation. This is found when two particles of charge q are separated by some distance r, i.e.:

The dipole moment, μ is:
μ = q.r
In a ferroelectric material, there is a net permanent dipole moment, which comes from the vector sum of dipole moments in each unit cell, Σμ. This means that it cannot exist in a structure that has a centre of symmetry, as any dipole moment generated in one direction would be forced by symmetry to be zero. Therefore, ferroelectrics must be non-centrosymmetric. This is not the only requirement however. There must also be a spontaneous local dipole moment (which typically leads to a macroscopic polarisation, but not necessarily if there are domains that cancel completely). This means that the central atom must be in a non-equilibrium position.
1.1 Ferroelectricity of perovskites
————————————————
Parnashi Chakraborty is currently pursuing Bachelor’s degree in Electrical
Engineerinr in the National Institute of Technology, Durgapur, PH- 91-
9836682811, Email-parnashi@gmail.com
Quite a lot of Ferroelectric materials are based on perovskite structure and hence at this point, let us highlight some aspects.
The general chemical formula for perovskite compounds is ABX3, where 'A' and 'B' are two cations of very different sizes built up of a Face centred cube and body centred cube. The ‘B’ cation is at the centre of the cube ‘A’ ions are larger than the
'B' ions. The ideal cubic symmetric structure has A cation in
12-fold cuboctahedral coordination. The relative ion size requirements for stability of the cubic structure are quite stringent; so slight buckling and distortion can produce several lower-symmetry distorted versions, in which the coordination numbers of A cations, B cations or both are changed.
Tilting of the BO6 octahedra reduces the coordination of an undersized A cation from 12 to as low as 8. Conversely, off-centering of an undersized B cation within its octahedron allows it to attain a stable bonding pattern. The resulting electric dipole is responsible for the property of ferroelectricity and shown by perovskites such as BaTiO3 that distort in this fashion.
1.2 Dielectric Constant versus Temperature
Another important property of these ferroelectric substances is that their dielectric constant increases as their temperature is increased. The dielectric constant of a material provides a measure of its effect on a capacitor. It is the ratio of the capacitance of a capacitor containing the dielectric to that of an identical but empty capacitor.
An alternative definition of the dielectric constant
relates to the permittivity of the material. Permittivity is a quantity that describes the effect of a material on an electric field: the higher the permittivity, the more the material tends to reduce any field set up in it. Since the dielectric material reduces the field by becoming polarised, an entirely equivalent definition is that the permittivity expresses the
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Current measurement using Ferroelectrics like Lead Bismuth Niobate and PZT 2
ISSN 2229-5518
ability of a material to polarise in response to an applied field. The dielectric constant (sometimes called the ‘relative permittivity’) is the ratio of the permittivity of the dielectric to
the permittivity of a vacuum, so the greater the polarisation developed by a material in an applied field of given strength, the greater the dielectric constant will be.
These ferroelectric materials are generally semi
conductor in nature. In case of semiconductors as temperature
increases resistance decreases. This is because at higher temperatures more electrons and holes are generated. Therefore at higher temperatures more dipoles are created, so permittivity increases and hence the dielectric constant as described above.
2. EXPERIMENT
2.1 Aim
The aim of the experiment is to measure current in a circuit using the property of ferroelectric materials that dielectric constant of some such substances change very steeply with temperature. The various characteristics of the ferroelectric material used have also been studied.
2.2 Principle
The ferroelectric compound is placed in a circuit. As current flows in the circuit the compound gets heated up due to is high resistance (being of semiconductor nature). This in turn leads to an increase in temperature of the compound. Now, as temperature increases its dielectric constant also increases. And the dielectric versus temperature graph is quite steep for most ferroelectrics i.e. for a very small change in temperature the change in dielectric constant is very high. ( this makes the measurements sensitive). Also resolution is very high. Thus by noting the change in dielectric constant the amount of current in the circuit can be predicted.
.
2.3 Equations involved
• D ∞ T D- dielectric constant
or, ΔD ∞ ΔT T- temperature
• I2Rt = msΔT I- current
R- resistance
• ΔD ∞ I2Rt/ ms m- mass
s- specific heat capacity
• √ΔD ∞ I t- time
Here dielectric constant can be considered proportional to temperature within a certain range of temperature which is different for each ferroelectric. Hence
change in dielectric constant is also proportional to the change in temperature over that range. I2Rt is the heat generated in the ferroelectric substance used due to the flow of current in it. Using msΔT we see the rise in temperature due to this heat. Thus we see that square root of the change in dielectric constant is proportional to the amount of current flowing in the circuit. And the proportionality constant is Rt/ ms.
The ferroelectrics used for the experiment are lead Bismuth Niobate (PbBi2Nb2O9) and Lead Zirconium Titanate (PbZrxTi1-xO3) or PZT based on perovskite structure.
2.4 Preparation of PbBi2Nb2O9
The preparation of lead bismuth niobate follows the equation given below:-
PbO + Bi2O3 + Nb2O5 → PbBi2Nb2O9
For 4 gms. of the material, 0.982g of PbO, 2.049g of Bi2O3 and
1.113g of Nb2O5 are taken in an agate mortar after weighing in
a Mettler electronic balance (Model AE-240) weighing accurately up to 0.1 mg.
The mixture is ground in a mortar pestle for two purposes

To convert the mixture into fine powder
To make a uniform mixture
During grinding, isopropyl alcohol was added to the mixture for more uniform mixing.
Now this mixture is placed in an alumina(Al2O3) container,
with the top covered and then placed in a tubular furnace. Heating and cooling were done in four steps which are as follows :
Temperature range (in degree C) | Time(in minutes) | Rate(in degrees per min) |
RT-800 | 80 | 10 |
At 800 | 360 | 0 |
800-500 | 120 | 2.5 |
500-100 | 120 | 3.33 |
After this, the compound was taken out of the furnace. Due to cooling, it formed a lump. Now again it was powdered by grinding using a mortar and pestle.
Making compact pellets using a hydraulic press.
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Current measurement using Ferroelectrics like Lead Bismuth Niobate and PZT 3
ISSN 2229-5518
Pellets have a diameter of 8mm and are about 2mm thick. Four tonnes of pressure was applied on them using the hydraulic press for about 5 to 7 minutes. A little poly vinyl
alcohol (PVA) known as binder was added to the powder before putting it under the hydraulic press so that compact pellets are formed and they don’t stick to the container. Sintering
For sintering the pellets are kept on an alumina crucible
placed on a brick which is made of refractory material (inert and has high structural integrity).then the entire setup is covered with a crucible made of alumina (which is also inert and has high thermal stability) to avoid escape of lead vapours. Then the edges of the crucible are sealed with heavy MgO and then placed in a programmable temperature controlled box furnace with the following temperature programme:
After synthesis, the compounds in powder form were characterised with respect to crystal structure by powder X- Ray Diffraction (XRD).
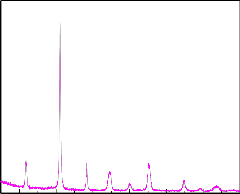
2.6 X-Ray Diffraction
rhombohedral tetragonal
Figure 1

20 30 40 50 60 70 80
2
It is mainly done for the completion of the reaction. It also provides time for grain growth which increases the breakdown voltage.
The cooled sintered pellets were taken out of the furnace and one of them was polished using emery paper.(only the two faces). The two polished faces were cleaned using iso- propanol and tissue paper. Then the two faces were silvered using a silver sol(silver dispersed in polymer). This was done to make the two surfaces conducting which is required for the dielectric constant measurements.
The silvered pellet was placed in the box furnace and kept at
5000C for 5 minutes for annealing and diffusion of silver in the
matrix of the compound synthesised.
2.5 Preparation of lead zirconium titanate(PZT)
The formula of PZT is Pb[ZrxTi1-x]O3. It was synthesised from PbO, ZrO2 and TiO2 by the usaula solid state reaction as before.
PbO + xZrO2 + (1-x)TiO2 → Pb[ZrxTi1-x]O3
The sample used in this experiment has x=0.9
In this case it is calcined in a furnace at 10000C for three
hours. And the sintering temperature is 11500C for six hours.
Figure 1 demonstrates the XRD pattern of PZT. It shows the coexistence of rhombohedral and tetragonal phases. The
rhombohedral phase less symmetric compared to the tetragonal is expected to be ferroelectric.
2.7 DSC Measurements
The sample of PbBi2Nb2O9.was characterised by Differential Scanning Calorimetry (DSC) to get an indication of the phase changes. In DSC there is one reference crucible and one sample crucible both made of platinum (inert and high melting point) placed on a block which is heated at a programmable rate. There is a differential thermocouple one end of which is connected to the reference crucible and the other end to the sample crucible. The temperature difference at this junction gives rise to a voltage at the junction due to seebeck effect. This voltage signal generated gives us an idea of the difference in power consumed by the reference crucible and the sample crucible. We get a power versus time graph. This power is, as stated not the absolute power consumption of any crucible but the difference in the power consumed by the reference crucible and the sample crucible. If the sample undergoes an exothermic process the graph shows us a peak and the opposite in case of an endothermic process. If we integrate the area covered by the peak with the time axis we can get the heat generated in the process. In our case we heated the sample from 300C to 7000C at the rate of
50/min. Any phase transition is associated with evolution or
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Current measurement using Ferroelectrics like Lead Bismuth Niobate and PZT 4
ISSN 2229-5518
absorption of heat which is indicated in the power versus time graph of DSC.
In the DSC measurement of PbBi2Nb2O9, we did not
observe any significant phase transition from room temperature till 7000C.
2.8 Dielectric vs Temperature Measurement
9000
8000
7000
6000
5000

' 4000
3000
2000
1000
100 Hz
1 KHz
10 KHz
100 kHz
For the applied voltage V the change in dielectric constant of the ferroelectric material and the corresponding current flowing in the circuit I is as shown in the table above.
igure 2
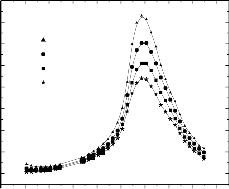
250 300 350 400 450 500 550 600 650 700
Temperature(K)
F 3. CONCLUSION
3.1 Reasons for using PZT or PbBi2Nb2O9
The dielectric constants of PZT were measured from room
temperature to 6500C at different frequencies from 100Hz to
The main reason for using PbBi Nb O
is that its
2 2 9
100KHz and shown in Figure 2. It is observed that there is a
sharp rise in dielectric constant from 450-5500C.
G. Details of the PZT sample used
WEIGHT OF THE SAMPLE: 0.4620g
AVERAGE THICKNESS: 1.53X10^-3 m
dielectric constant versus temperature graph has a very steep slope i.e. for a very small change in temperature the change in dielectric constant is very high. Therefore the resolution will be very high. According to calculation the slope is approximately 82 degrees.
For PZT the difference between two readings of
current can be of the order of 10-6 and it is similar for
PbBi Nb O . Both these compounds have very high
2 2 9
AVERAGE DIAMETE: 7.335X10^-3 m
RADIUS: 3.6675X10^-3 m
AREA: 4.225653X10^-5 m2
Dielectric constant D = 66.67 T (where T is the temperature)
approximately
Calculated Data:
breakdown voltages. Also the range of temperature over which the steep change takes place is pretty high, about 400.
The main reasons for using PZT are that firstly, the temperature values over which the dielectric constant change takes place is low comparatively. Hence it gets heated up at low current. Secondly according to the XRD analysis it has a mixed rhombohedral and tetragonal phase. While titanium rich compositions are in tetragonal phase, the zirconium rich are in rhombohedral phase. Therefore it has asymmetry in structure which gives better ferroelectric and piezoelectric properties.
3.2 Advantages of using this method of current measurement and its Application.
ΔT | I(calculated) | V(required) | ΔD |
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Current measurement using Ferroelectrics like Lead Bismuth Niobate and PZT 5
ISSN 2229-5518
This technique of current measurement can be used to measure leakage current in insulators when placed in series with the insulators in the circuit. Also current
through ferroelectric materials which is a semiconductor can be easily measured. The main advantage of using this method is the fact that the resolution is very high. The least count can be as low as 10^-6. Sensitivity is also high.
4. ACKNOWLEDGEMENT
I would like to thank my mentor Dr. S.K. Bandyopadhyay for his help, support and constant guidance without which this project would have been impossible. I would also like to thank the head of the Physics department of Variable Energy Cyclotron Centre(VECC), Dr. D.K. Srivastava for giving me the opportunity to work in their well equipped laboratories.
Last but not least I am grateful to my parents for their
constant guidance and my friend Esha Sarkar for her encouragement and support.
5. REFERENCES
[1] Marin Alexe, ‚Ferroelectric material for piezoelectric, pyroelectric, and memory applications, Nanoscale ferroelectrics”, Max Planck Institute of Microstructure Physics, Halle-Germany.
[2] Jinhong Bi, Ling Wu, Zhaohui Li, Xuxu Wang and
Xianzhi Fu, ‚ A citrate complex process to prepare nanocrystalline PbBi2Nb2O9. At a low temperature.‛Elsevier, Materials letters Volume 62, Issue
1, Pages 155-158.
[3] Tao Zeng, ZianLin Dong, Hong Yang, ChaoLiang Mao
and Heng Chen, ‚ Enhancement of mechanical and dielectric breakdown properties by diffusion of SiC into lead zirconate titanate ceramics, Elsevier, Scripta Materialia 55 (2006) 923-926.
[4] Junzhong Wang, Yuan Hu, Rui Zgang, Lei Song, Zuyao
Chen, ‚ Sonochemical preparation of net-lead zirconate titanate(PZT)‛, Elsevier, Journal of Crystal Growth 263 (2004) 377-384.
[5] A K Himanshu, d C Gupta, Alo Dutta, T P Sinha, S K
Bandyopadhyay, ‚ Structural and Dielectric behavior of
Barium substituted lead zinc niobate ceramics at low temperature‛, Indian Journal of Pure and Applied Physics, Vol. 47, March 2009, pp. 212-219.
[6] B. Sahoo, V A Jaleel, P K Panda,‛ Development of PZT
powders by wet chmical method and fabrication of
multilayered stacks/actuators‛, Elsevier.
IJSER © 2012 http://www.ijser.org

