International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 1
ISSN 2229-5518
Cryopreservation of Human Sperm: Effect of
Cooling Rate on Intracellular Ice Formation
D.Devismita, A.Kumar, R.KrishnaKumar
————————————————————
istory suggests that sperm cryopreservation had started
in the late 1950’s and in the last few decades there have been enormous developments in the field of its preservation at low temperatures [1],[2],[3]. It became a key area of scientific investigation when the dairy industry needed long term storage methods for preservation of bull sperm[4].This area of research came into prominence only when the foundation of human sperm cryopreservation was laid by Polge et al in 1949 discovering the use of glycerol as a cryoprotective agent and that it could provide protection to the cells at low temperature [5].In the early nineteenth century Montegazza, discovered that human sperm can actually survive at temperatures below
−15°C but the majority of these sperms could not survive the
damaging effect of ice formation(i.e. freezing injury). The supplementary deciding factors for thriving cryopreservation are the sperm dilution rate, cooling rate, thawing rate and the
————————————————
![]() Dr Amitesh Kumar is currently Assistant Professor in Biotechnology and
Dr Amitesh Kumar is currently Assistant Professor in Biotechnology and
![]() R. Krishna Kumar is currently pursuing PhD degree program in
R. Krishna Kumar is currently pursuing PhD degree program in
Biotechnology and Medical Engineering in National Institute of Technology, Rourkela, India, PH-09439734986 ,
E-mail:krishnakumar.ramajayam@yahoo.com
rate of intracellular ice formation, which is the main cause of cellular damage. The ice formation is a significant and detrimental factor which affects cryopreservation. Better understanding of intercellular ice formation rate will eventually lead to the production of many viable sperm cells. Also, apart from the change in the rate of intercellular ice formation a successful sperm cryopreservation requires maintaining the post-thaw structural and functional integrity [6],[7],[8],[9],[10],[11].
Though there has been incessant progress in the field of sperm cryopreservation but still there are many questions to be answered and many baffling puzzles to be solved. Hence, the main objective of this study is to adjoin one more footstep for the proper preservation of human sperm by simulating the optimal cooling rate.
And this will quantify the effect of cooling rate on intracellular
ice formation for human spermatozoa cell lines. The
biophysical properties of human sperm are taken from the published literature [7].
The water transport during cryopreservation of a cell is driven by a chemical potential difference between the intracellular and extracellular solution. The intracellular water moves towards the extracellular solution which leads to the volumetric shrinkage of the cell as a result of this potential difference. Mazur [8] proposed a theoretical model for water transport across the cell membrane which was later modified by Levin et al. [9] and is given as,
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering RESEARCH, VOLUME 3, Issue 6, June-2012 2
ISSN 2229-5518
![]()
![]()
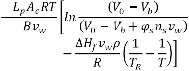
TABLE 1
BIOPHYSICAL PROPERTIES FOR HUMAN SPERM FROM DEVIREDDY ET ![]() AL. [7].
AL. [7].
![]()
![]()
![]()
![]()
Where Lp is the sperm membrane permeability to water, given as below
Symbol Value Unit
![]()
TR 273.15 K
r0 0.42 μm L 40.2 μm Ac 106 μm2
Lp is an Arrhenius function of Lpg and![]() .
.
The present numerical code is validated against the published experimental result of Devireddy et al. [7]. In the experiment, the volumetric response of human sperm as a function of subzero temperatures was obtained using the differential scanning calorimeter technique. Figure 1 shows a comparison between numerically computed volumetric response of human sperm and the experimental measurements.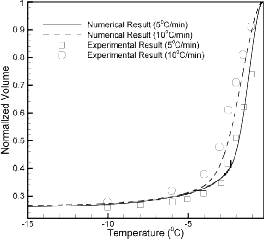
Fig1. Validation of the present numerical code.
V0 22.2 μm3
Vb 0.23 V0
R 8.314 J/mol K
vw 18Х1012 μm3/mol
∆Hf 335 mJ/mg
![]()
In this study, the human sperm is modeled as a long cylinder with biophysical properties given in Table 1. The osmotically inactive cell volume (Vb) is assumed to be 0.23V0. The values of two membrane permeability parameters are taken as the best fit parameters obtained from the published results. To assess the effect of cooling rate on intracellular ice formation the numerical results are obtained for different cooling rates varying from 5°C/min to 300°C/min. It has been assumed that the amount of water trapped inside the human sperm at subzero temperature of -19°C will finally form the intracellular ice with sufficient super-cooling. (In the figure, the cooling rate is increasing from 5⁰C/min to 300⁰C/min as we proceed from the bottom to top)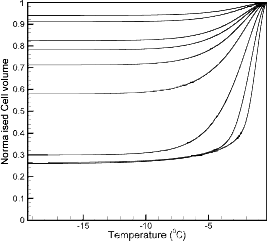
The rectangles and circles represent experimental values for a
cooling rate of 5°C/min and 10°C/min respectively and the
solid line and dotted line represent the numerical result for the
cooling rates of 5°C/min and 10°C/min respectively. The
numerical results match quite well with the experimental
results validating our numerical model.
Figure 2. Normalized cell volume at different cooling rates varying from
5⁰C/min to 300⁰C/min.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering RESEARCH, VOLUME 3, Issue 6, June-2012 3
ISSN 2229-5518
Figure 2 shows the volumetric response of human sperm for cooling rates of 5°C/min, 10°C/min, 20°C/min, 40°C/min,
60°C/min, 80°C/min, 100°C/min, 200°C/min, 300°C/min (from bottom to top). As the cooling rate increases the amount of water trapped inside the cell increases till the higher cooling rate is reached, after which there is a constancy in the volume of intracellular water. It should be noted that up to a
certain cooling rate (termed as optimal
cooling rate in this paper) all the water inside the cell leaves
the intracellular compartment leaving behind the osmotically inactive cell volume as the end volume. The value of the optimal cooling rate is found to be nearly equal to 17°C/min. After the optimal value the trapped water volume increases with increase in the cooling rates as reflected in the figure. Also, this increase is not linear with the increase in cooling
rate.
TABLE 2 NOMENCLATURE
![]()
V0 Isotonic sperm volume vw Molar volume of water
Vb Osmotically inactive sperm volume ∆Hf Latent heat of fusion of water
Lpg Reference membrane permeability
ELp Apparent activation energy
Ac Effective membrane surface area
Greek Symbols
φ Disassociation constant
L Sperm length, mm ρ Density of water, m3
R Universal gas constant (J/molK) Subscripts
TR Reference temperature (K) s salt
B Cooling rate (K/min) w water
![]()
Fig 3. Correlation between the cooling rate and the volume of intracellular ice formation.
Figure 3 shows the correlation when the cooling rate and the normalized trapped water. The plot is showing a curve which increases linearly till the optimal cooling rate and thereafter it increasing exponentially with the increase in cooling rate. This indicates that, after a certain range of cooling rate called as optimal cooling rate the volume of trapped water is increasing with increase in cooling rate by forming more amount of intracellular ice. If the cooling rate is much higher than the optimal value then water inside the cell will not get sufficient time to exit and will form ice within the cellular environment which may either lead to crystal formation or rupture of the cell wall and other cellular injuries.
With the numerical data obtained for intracellular ice
volume, a correlation formula is established between the cooling rate and the volume of intracellular ice formation to justify the effect of cooling rate on intracellular ice formation for a human sperm. The correlation is described
by the following equation:
IJSER © 2012 http://www.ijser.org
![]()
![]()
![]()
Where a=0.6904, b= -0.9916, c= 0.0251 min/0C and Vw= volume of water trapped, Vw0= initial water volume, CR = cooling rate.
A correlation formula between the cooling rate and the volume of intracellular ice formation was obtained for human sperm in this study. The intracellular ice formation during different cooling rates was studied within a range from 5°C/min to 300°C/min. It was found that after a certain cooling rate called as optimal cooling rate (which is
~17°C/min in this case) the intracellular ice formation
increases exponentially. The correlation established in the current study will help in finding the optimal cooling rate for other mammalian sperm cells as they exhibit almost
same biophysical properties during freezing and this will enhance our knowledge for optimizing equine sperm cryopreservation along with other mammalian sperm cells.
[1] L.Spallanzani, ―Osservation e spezien interno ai vermicelli spermatici dell’ uomo e degli animali‖, In opusculi di fisica Animale e Vegatabile, Opuculo II. Modeena, Italy (cited in Brotherton, 1990), 1776.
[2] C.B.Davenport, ―Experimantal morphology: part I, Effect of chemical and physical Agents upon Protoplasm‖, Macmillan Company, New York, 1897.
[3] A.S.Parkes, ―Preservation of Human spermatozoa at low
temperatures‖, Br Med J., ii,212-213,1945.
[4] J.K. Sherman,―Synopsis of the use of frozen human semen since 1964: state of the art of human semen banking. Fertil
Steril‖, 24, 397–412, 1973.
[5] C.Polge,A.U.Smith, and A.S.Parkes, ―Revival of spermatozoa after vitrification and dehydration at low temperatures‖, Nature,164, 666–668, 1949.
[6] E.M.Walters, J.D.Benson, E. J. Woods, and J.K. Critser,―The history of sperm cryopreservation‖, Sperm banking: Theory and Practice, Cambridge University Press, 2009.
[7] R.V.Devireddy,D.J.Swanlund,K.P.Roberts,J.L.Pryor, and J.C.Bischof, ―The effect of EIF and cryoprotective agents on the water permeability parameters of human sperm plasma membrane during freezing‖, Human Reproduction,Vol.15, no.5, pp.1125-1135,2000.
[8] P.Mazur, ―Kinetics of water loss from cells at subzero temperature and likelihood of intracellular freezing‖, J.Gen. Physiol, 47,347-369,1963.
[9] P.Mazur, S.P.Leibo, and E.H.Y.Chu, ―A two-factor hypothesis of freezing inzury‖, Exp cell Res, 71,345-355,1972
[10] P.Mazur, ―Freezing of living cells: mechanisms and
implications‖, Am J Physiol,247,C125–C142,1984.
[11] P.Mazur, ―Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos‖ [Review], Cell Biophys,17,53–92,1990.
[12] R.L.Levin, E.G.Cravalho, and C.G.Huggins,―A membrane model describing the effect of temperature on the water conductivity of erythrocyte membranes at subzero temperatures‖, Cryobiology,13,415–429,1976.
[13] D.Y.Gao, P.Mazur, and J.K.Critser, ―Fundamental cryobiology of mammalian spermatozoa‖, In: Karow AM, Critser JK (eds.), Reproductive Tissue Banking. San Diego: Academic Press,263–328,1997.
[14] O.Kedem, A.Katchalsky, ―Thermodynamic analysis of the permeability of biological membranes to non-electrolytes‖, Biochim Biophys Acta,27,229–246,1958.
[15] M.Toner, ―Nucleation of ice crystals in biological cells‖, In: Steponkus PL (ed.), Advances in Low-Temperature Biology, Greenwich, CT: JAI Press,vol. 2,1–52,1993.
IJSER © 2012 http://www.ijser.org