International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 336
ISSN 2229-5518
Concentration Technique during the recovery of alkaline phosphatase from Red Shrimp
Hepatopancreas.
Krishna Prasad Nooralabettu
Professor, P. A. College of Engineering, Nadupadavu, Mangalore, Karnataka, Mangalore, India, Pin-574153, E-mail: lodhariad1@hotmail.com, Phone: +91-9448529048
Abstract— Alkaline phosphatase was released from the Red shrimp hepatopancreas by homogenisation at 3,000 rpm for 10 min at
4oC, and the homogenate was clarified at Relative Centrifugal Force of 1681.1×g for 5min at 4oC. The efficiency of pH and ammonium sulfate saturation to concentrate the enzyme with optimum yield determined by subjecting the homogenate to 15, 25, 35, 45, 55, 65,
75 or 85% saturation level at 0oC in 0.1 M Tris-HCl buffer of pH 8.4 or 2 M KCl solution of pH 7. The saturation level of 65% at pH 8.4
was able to efficiently precipitate 92.63±0.06% of the alkaline phosphatase into one third of the bulk volume resulting in 7.55±0.04 folds purification, and retain half of the protein impurities in two third of the bulk volume. W hereas, the enzyme yield was reduced below 65% saturation level and enzyme purification was reduced above 65% saturation level.
Index Terms— Alkaline phosphatase, Red shrimp, Ammonium sulfate, Precipitation, Concentration, Relative Centrifugal Force.
—————————— ——————————
1 INTRODUCTION
rotein impurities encountered during the purification of alkaline phosphatase from shrimp hepatopancreas
are the bottle neck to commercially exploit it, because it is a crucial organ plays an important role in food absorption, transport, secretion of digestive enzymes, and storage of lipids, glycogen, and a number of minerals (McComb et al., 1979; Hanpongkittikun et al., 1995; Chuang and Yang, 1990). Is very much important to Reduce the bulk volume of the homogenate and concentrate the alkaline phosphatase by exploiting physico-chemical properties of the components of the homogenate by ammonium sulfate precipitation at the initial stage of the purification itself to stabilize the partially purified alkaline phosphatase, and to reduce the space requirement, associated ingredients demand and cost of purification (Nooralabettu, 2011, Arakawa and Timasheff, 1985). Various research publications were discussed the importance of parameters of ammonium sulfate precipitation during protein recovery, but no laboratory models were designed to identify discriminative specific parameters critical for the isolate alkaline phosphatase from other protein impurities of the pancreas with similar physico-chemical properties (Chiew et al., 1995; Hatti-Kaul and Mattiasson, 2003; Wheelwright, 1991). Lower saturation level of ammonium sulfate precipitates proteins with few hydrophilic regions and at higher saturation level some other proteins with more hydrophilic region are precipitated without changing its native conformation of the precipitate (Nooralabettu, 2012; Aukhil et al., 1993; Doonan and Cutler 2004; Mirica, 2012; Ko and Ahn, 2007; Moore and Kery, 2009). In one hand, no single set of standard operating conditions required to concentrate the
enzyme from the clarified tissue homogenate of the shrimps with reference to the requirement of ammonium
saturation level and pH have been defined. On the other hand, the saturation level of the ammonium sulfate required to precipitate various proteins in the homogenate varies from one protein to other protein under given condition and various external factors influence the pattern of protein precipitation, we have made an effort to investigate the ammonium sulfate saturation and pH level required to remove maximum quantity of contaminating proteins and water from the enzyme mixture optimally using a standard set of operating parameters to isolate alkaline phosphatase from Red shrimp (Solenocera choprai) hepatopancreas with optimum yield and activity.
1. Materials and Methods
2.1 Chemicals Analytical grade chemicals and reagents
obtained from Merck Limited (Mumbai, India) and solutions were prepared according to the current American Chemical Society specifications (ACS, 1999). Homogenisation and precipitation of hepatopancreatic tissues of the shrimp was carried out using Tris-HCl buffer or KCl solution. Working buffer, 0.1 M Tris-HCl buffer of pH 8.4 was prepared by adding 12.111 g of the free Tris base to 900 mL of deionized water. This mixture is then titrated with 1 M HCl solution to pH 8.4, and volume was made up to 1,000 mL. The buffer was added with MgCl2 and ZnCl2 to respective final concentration of
1 mM. Whereas, 2M Potassium chloride (KCl) solution
was prepared by dissolving 149.1 g of potassium chloride
using deionised water, and pH was adjusted to 7 using
0.1 M NaOH solution. Assay buffer, 2-amino-2-methyl-1-
propanol (AMP) buffer of pH 10.3 was prepared by
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 337
ISSN 2229-5518
dissolving 78 g of AMP in 500 mL of deionized water. To this resulting mixture 200 mL of 1 M HCl is added. Subsequently, volume of the mixture is made up to 1,000 mL using deionized water in 1,000 mL volumetric flask. Assay buffer is used to determine the alkaline phosphatase activity. All the buffers were filtered and sterilized at 121oC for 20 min (Puttige and Nooralabettu,
2011).
2.2 Sample collection During the month of July and
December Red shrimp (Solenocera choprai) were caught using trawl nets from the Arabian Sea and samples were obtained from the fishing boats landed in ‘Bunder area’, Mangalore The time elapsed between catching and landing may not exceed over four to six hours. The material was kept in an insulated container after adequately icing them in the proportion of 1:1 shrimp to ice, and transported to the laboratory within two hours. Red shrimp available along the coastal Karnataka was identified and used for the present study (Racek, 1955). Freshly caught Red shrimp with the weight range of 20-
25 g were washed and dissected to remove the
hepatopancreas. The hepatopancreas and attached tissues
were sorted out, and weighed. Hepatopancreatic tissues were packed in plastic bags, labeled, frozen at –40oC, and stored at –20oC in a deep freezer (JHBio, Chennai, India) until further use.
2.3 Homogenization The samples so prepared was
thawed at room temperature of about 28oC, weighed and homogenised using a Potter-Elvehjem homogenizer (RH-
2 Homogenizer, Rotek Instruments, Kerala, India) with a sample holding tank mounted in a cooling jacket. The tissues were homogenized in the homogenizer at homogenization speed of 3,000 rpm for 10 min at the temperature of 4oC using 0.1 M Tris-HCl buffer of pH 8.4 or 2 M KCl solution of pH 7 at 1:10 tissue to buffer ratio (Puttige and Nooralabettu, 2012).
2.4 Centrifugation The samples with highest protein
content and alkaline phosphatase activity were centrifuged at relative centrifugal force (RCF) of 1681.1×g for 5 min at 4oC in C-24BL/CRP24 model microprocessor controlled low volume high speed refrigerated centrifuge (Remi Laboratory Instruments, Mumbai, India) (Nooralabettu and Puttige, 2013).
2.5 Ammonium sulfate precipitation Ammonium sulfate
crystals were dried overnight at 120oC and ground finely using pestle and mortar. Infranatant obtained after centrifugation were taken in 3 mL centrifugation tubes and placed in a cooling jacket maintained at 0oC. While keeping the samples in ice jacket, 0.066, 0.144, 0.209,
0.277, 0.351, 0.430, 0.516, or 0.608 g/mL of finely ground ammonium sulfate crystals were added gently with intermittent agitation to dissolve all the added crystals to achieve 15, 25, 35, 45, 55, 65, 75 and 85% saturation level, respectively. This step took around 15 min and the mixture was further stirred for 20 min at 0oC. Subsequently, mixture was centrifuged at 15,124.8×g for
30 min at 0oC. Supernatant was decanted and precipitate
was reconstituted in 0.1 M Tris-HCl buffer of pH 8.4 or 2
M KCl solution of pH 7 at 1:1 pellets to buffer ratio, and
samples were estimated for protein content and alkaline phosphatase activity. The ammonium sulfates from the precipitates were removed by the process of dialysis.
2.6 Dialysis Dialysis tube of 10 kDa was prepared by
boiling the tubes in 10 mM sodium bicarbonate containing 1mM EDTA for 20-30 min. The tubes were cooled and washed extensively in distilled water, and was stored at 4oC. One end of the dialysis tube was closed using leak proof clamps (Himedia, Mumbai). Respective reconstituted pellets were dispensed into the tubing using pipette. The other end of the dialysis tubes were clamped with clamps while keeping sufficient space above the sample and placed in the beaker containing more than ten times volume of the 0.1 M Tris-HCl buffer of pH 8.4 maintained at 4oC. Magnetic stirrer was used to stir the buffer gently to improve solute exchange. The dialysis buffer was changed once in three hours. Respective samples were drawn at 6, 12, 16 and 24 h of dialysis and estimated for total protein content and alkaline phosphatase activity.
2.7 Proximate analysis Samples were drawn at different
intervals of experiment was performed in quadruplicates. The protein content was estimated as per the Folin- Ciocalteau method (Lowry et al., 1951), using bovine serum albumin (BSA) as a standard. Total protein content of the hepatopancreatic tissues were done by incubating
0.4 mg of tissues with 0.5 mL of 4 M NaOH at 100oC for 5
min, and the resulting homogenate was cooled and assayed for total protein by Folin-Ciocalteau method. Similarly, protein content in the hepatopancreatic tissue homogenate during homogenization or in three phases after centrifugation was estimated using Folin-Ciocalteau method.
2.8 Enzyme assay Alkaline phosphatase was assayed
using disodium paranitrophenyl phosphate as a substrate (Bowers and McComb, 1975). The substrate was prepared by dissolving 83.5 mg of disodium paranitrophenyl phosphate (pNPP) in 1.0 mL of 1.5 mM magnesium chloride solution and stored at 4oC. This solution was colourless and its absorbance was measured at 410 nm<
0.800. A stock solution of 10.8 mM/L of pNP was
prepared by dissolving 150 mg of pNP in about 80 mL of
0.25 M NaOH solution and stored at room temperature of about 28oC in amber colored bottle. A working solution of
54 mM/L of pNP was freshly prepared by pipetting 0.5 mL of pNP stock solution in 100 mL volumetric flask and the volume was made up to the mark using 0.25 M NaOH solution. Enzyme assay incorporates AMP buffer. About
1.4 mL of buffer was mixed with the solution and
incubated at 37oC for 5 min. Then 0.05 mL of the hepatopancreatic tissue homogenates was added. To this mixture, 0.1 mL of the substrate was added, mixed and incubated at 37oC for 15 min. Then, 4 mL of the 0.25 M NaOH was added to each tube in sequence maintaining timed intervals to terminate enzyme activity. Then, the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 338
ISSN 2229-5518
solutions were mixed and cooled to room temperature (28oC). Colourless pNPP gets hydrolyzed by alkaline phosphatase at a given buffer pH and incubation temperature of 37oC to form yellow colored free pNP, which shows maximum absorbance at 410 nm in a spectrophotometer that was set to zero with the blank. In our alkaline phosphatase assay, 0.05 mL of tissue homogenate was mixed with reagent and incubated for
15 min and the total volume was made up to 5.55 mL.
However, the total volume in the case of each standard was 5.0 mL. Hence, pNP in mM/L or alkaline phosphatase activity in units/L in the tissue homogenate = (Test absorbance × 0.027 × 5.55 × 1,000)/ (Standard absorbance ×
15 × 5.0 × 0.05). Alkaline phosphatase activity in units/L is
the liberation of 1 mM of pNP per min at 37oC incubation temperature per liter of tissue homogenate in respective buffers. We made no corrections for the slight variation of molar absorptivity of pNP with pH and (or) buffer concentration.
2.9 Statistical analysis The analysis of alkaline
phosphatase recovery was carried out in quadruplicate. The results were treated by analysis of variance (ANOVA), followed by Tukey’s test, using the software Statistica 6.0 (Statsoft, Tulsa, OK, USA). The results were expressed as averages ± standard deviations followed by corresponding letters which indicates the significant differences. All analyses were performed considering a confidence level of 95% (p<0.05).
2. RESULTS AND DISCUSSION
Clarified hepatopancreatic tissue homogenates of Red shrimp were subjected to ammonium sulfate precipitation at various saturation levels under various conditions to determine the effectiveness each of these parameters to reduce the bulk of the medium and improve the enzyme yield.
3.1 Optimisation of pH during ammonium sulfate
precipitation At pH 8.4, alkaline phosphatase activity in the pellets was 2.68±0.01, 2.86±0.01, 2.86±0.01, 9.86±0.02,
73.24±0.02, 98.25±0.03, 98.25±0.02, and 96.54±0.03% activity of its initial homogenates, respectively, respectively, at ammonium sulfate saturation level of 15,
25, 35, 45, 55, 65, 75, and 85% (Fig. 1). However, at pH 7, the saturation level of 15, 25, 35, 45, 55, 65, 75 and 85% was able to fraction only 11.47±0.01, 3.83±0.01, 5.10±0.01,
15.95±0.09, 24.88±0.07, 24.24±0.07, 14.04±0.07, and
12.76±0.08% of the alkaline phsophatase of its respective
homogenate, respectively. At pH 8.4, residuel alkaline phophatase activity of the supernatant was only
44.15±0.06, 37.64±0.06, 27.43±0.05, 28.71±0.06, 19.14±0.01,
10.85±0.02, 7.02±0.02, and 3.19±0.01% at pH 7, whereas the
activity was 97.32±0.06, 97.14±0.06, 97.14±0.06, 90.14±0.05,
26.76±0.06, 1.75±0.009, 1.75±0.023, and 3.54±0.014% of its initial homogenate, respectively, at 15, 25, 35, 45, 55, 65,
75 and 85% saturation level. Pellets obtained at these saturation levels at pH 7 were showing lower levels of alkaline phosphatase activity in comparision to the pellets
obtained at the same saturation levels of ammonium sulfate but at pH 8.4. The overall significant effect of pH of the medium on alkaline phosphatase activity of the pellets at all saturation level remained at 5% level of significance, as indicated by one-way ANOVA with post hoc Tukey’s test. Here, alkaline phosphatase activity of the pelletes obtained at a given ammonium sulfate saturation level is influenced by the pH of the precipitation medium (Ko and Ahn, 2007).
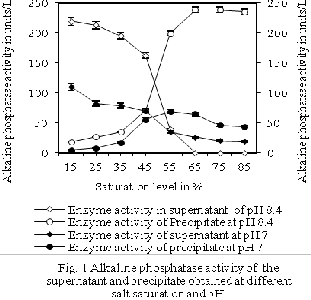
When the precipitation was carried at pH 8.4, increase in ammonium sulfate saturation level from 15% to 65% significantly (p<0.05) increased the precipitation of alkaline phosphatase into the pellets. Here, the results of analysis of variance with p-values demonstrate significance for the regression model for both ammonium sulfate saturation level and precipitation of alkaline phosphatase. Beyond 65% saturation at pH 8.4 one-way ANOVA with post hoc Tukey’s test was not able to establish a significant difference in the alkaline phosphatase activity amongst the pellets obtained at different saturation. Previous work shows that ammonium sulfate at 60% saturation level efficiently removes High molecular weight proteins (Akita and Nakai, 1992). Here at pH 7, increase in the ammonium sulfate saturation level from 15 to 55% significantly (p<0.05) increased the precipitation of the proteins with alkaline phosphatase activity into the pellets. Hoever, beyond 55% saturation level at pH 7 significant (p>0.05) decrease in the fractionation of the proteins with alkaline phosphatase activity took place in comparision to its activity at 55%. Alkaline phosphatase isolated from the hepatopancreas of Indian white shrimp shrimp was repoterd to have optimum pH of 8.4 (Puttige and Nooralabettu, 2011; Puttige and Nooralabettu, 2012; Nooralabettu and Puttige, 2013). Hence, components and the pH of the buffer used for ammonium sulfate precipitaion plays a major role in alkaline phosphatase activity, because of the three metal binding sites in the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 339
ISSN 2229-5518
active sites of the enzyme, two zinc ions are shown to play a direct role in catalysis (Bortolato et al., 1999). Hence, 0.1 M Tris-HCl buffer of pH 8.4 with MgCl2 and ZnCl2 to respective final concentration of 1 mM was used as a working buffer for subsequent precipitaion experiments.
3.2 Optimisation of ammonium sulfate saturation level
Total protein present in the clarified tissue homogenate of Red shrimp was estimated as 1213.00±63.89 mg/L, was subsequently fractioned between the pellets and supernatant at 15, 25, 35, 45, 55, 65, 75, and 85% ammonium saturation level as represented in the figure 2.
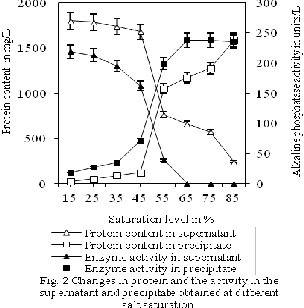
At 15, 25, 35, and 45% ammonium saturation level, only
2.23±0.02, 2.97±0.02, 5.44±0.01, and 11.13±0.02% of total protein was precipitated from the respective clarified homogenate, respectively. Neverthless, saturation levels of 55, 65, 75, and 85% ammonium sulfate respectively, precipitated 50.21±0.13, 53.92±0.12, 63.07±0.13, and
64.53±0.14% of the total protein present in the clarified tissue homogenate into pellets. Here, one-way ANOVA with post hoc Tukey’s test was able to establish significant (p<0.05) difference in the protein content of the pellets between the samples obtained at 15, 25, 35, 45, 55, 65, 75, and 85% the saturation level. This is due to some proteins with few hydrophilic regions precipitate at lower ammonium sulfate saturation level and some other proteins with more hydrophilic region precipitate higher saturation of ammonium sulfate saturation level (Mirica et al., 2012; Ko and Ahn, 2007). Protein precipitation by ammonium sulfate precipitation is high-throughput method compared to ultrafiltration because proteins in mixture can be separated from one another efficiently based on their relative hydrophilicity by gradually increasing the concentration of ammonium sulfate (Moore and Kery, 2009).
A the saturation level was increased from 15 to 55%, alkaline phosphatase activity in the pellets wereincreased from 2.68±0.06% to 98.25±0.06%, and subsequently
remained at a level of 97.65±0.29% of the activities in the clarified homogenate even up to 85% saturation level. one-way ANOVA with post hoc Tukey’s test was able to establish higher (p<0.05) level of factionation of the total protein and alkaline phosphatase into the precipitate at
55, 65, 75, and 85%, compared to 15, 25, 35, and 45%
saturation levels. Beyond 65% saturation level no
significant change (p>0.05) in the alkaline phosphates activity was registered among the precipitates, Eventhough increase in ammonium sulfate saturation level from 15% to 85% significantly (p<0.05) increased the precipitation of protein. This is due to ammonium sulfate saturation levels of more than 60% reduce substantial high molecular weight proteins and majority of the hydrophobic protein (Akita and Nakai, 1992; Kumar et al.,
2012). Here, beyond 65% saturation level, significant
(p<0.05) levels of contaminating proteins were also
fractioned into the precipitates when there was no significant change (p>0.05) in the alkaline phosphatase activity was registered in the precipitates as established by one-way ANOVA with post hoc Tukey’s test. The salt saturation level of 45% was able to precipitate only around one third of the alkaline phosphatase activity, and the saturation level 55% was able to precipitate only around three fourth of the activity, and at saturation level of 65% or more was able to precipitate more than 95% of the activty of clarified tissue homogenate. The optimum alkaline phosphatase and maximum protein precipitation was achieved at 20-50% fractions compared to 1-20% or
50-80% saturaion level (Ward and Swiatek, 2009). In one hand, the saturation level of the ammonium sulfate required to precipitate various proteins in biological mixture varies from one protein to other protein and should be to be done empirically, and on the other hand pH of the medium has a very important role to play.
3.3 Enzyme fractionation and concentration during
precipitation To study the efficiency of the saturation
level to retain maximum quantity of the contaminating proteins in the bulk of the volume and to fractionate maximum quantity of the insolubilised alkaline phosphatase into a minimal volume as possible resulting concentration and to some extent purification of the enzyme, we have used 818.53±0.81 mL of the clarified homogenates for precipitation experiment at 15, 25, 35,
45, 55, 65, 75, or 85% ammonium sulfate saturation levels. The salt saturation level of 15, 25, 35, 45, 55, 65, 75, and
85% was able to fractionate 97.63±0.14, 96.04±0.14,
95.05±0.07, 94.72±0.04, 87.92±0.09, 81.22±0.01, 81.22±0.05,
and 79.72±0.18% of the volume as a supernatant with
97.32±0.06, 97.14±0.06, 97.14±0.06, 90.14±0.05, 26.76±0.06,
1.75±0.009, 1.75±0.023, and 3.54±0.014% alkaline
phosphatase activity of its initial homogenate, respectively (Fig 3). Specific activity of the pellets significantly (p<0.05) reduced as the ammonium sulfate saturation level increases from 15% to 35%, as established by one-way ANOVA with post hoc Tukey’s test. Further increase in the ammonium sulfate saturation level
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 340
ISSN 2229-5518
significantly (p<0.05) increased the specific activity, even up to 65% saturation level.
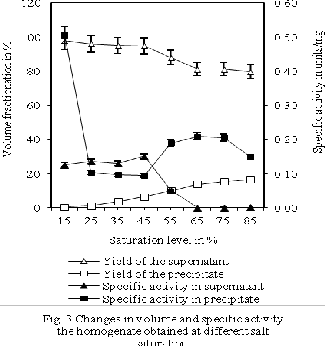
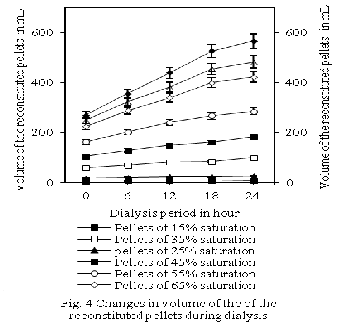
However, significant (p<0.05) fall in the specific activity was observed as the saturation level increased from 75% to 85%. Pellets exhibited highest specific activity for alkaline phosphatase at 15% saturation levels of ammonium sulfate, though registering lowest of the enzyme yield at this saturation level compared to the other pellets. Nevertheless, pellets obtained at 65% saturation level produced lower specific activity in comparison to the pellets obtained at 15% saturation level, but highest alkaline phosphatase activity amongst all the pellets. Here, highest specific activity in the pellets produce at 15% saturation level is due to the lowest protein content in the pellets compared to the precipitates produced at higher saturation level. Significant increase in protein precipitation even at insignificant change of alkaline phosphatase activity is responsible for the reduction in the specific activity beyond 75% saturation level. Optimum specific activity of alkaline phosphatase was observed at ammonium saturation level of 65%, and above and below this level specific activity was decreased, with the exception of at 15% saturation level. The clarified tissue homogenates of the shrimp divided into supernatant with more than four fifth the volume and precipitates with less than one fifth of the volume at these ammonium sulfate saturation level. However, volume of the supernatant increased as the saturation levels of the ammonium sulfate reduced from 85 to 15%. Volume of the reconstituted pellets nearly doubled during the dialysis of the reconstituted precipitates using ten times the volume of the 0.1 M Tris-HCl buffer of pH
8.4 at 4oC for 6, 12, 18, and 24 h, (Fig 4). No significant
change in the specific activity was observed in each
pellets obtained at different saturation levels, even when the volume of the samples doubled during the entire 24 h of dialysis of the reconstituted pellets. This is because during entire 24 h of dialysis the dilution of the reconstituted pellets resulted in simultaneous dilution of both total protein and alkaline phosphatase. Enzyme yield was only 2.53±0.09% of the initial homogenate used, even though the alkaline phosphatase was purified by
4.99±0.02 folds during the entire process of enzyme
recovery at 15% saturation level. Nevertheless, when saturation level was increased to 65% ammonium sulfate enzyme yield was increase to 92.63±0.06% and purification was increased by 7.55±0.04 folds of the initial tissue. One-way ANOVA with post hoc Tukey’s test was not able to establish significant (p>0.05) difference in enzyme yield and purification fold amongst the samples precipitated at 65, 75, and 85% saturation level. Hence, it makes more sense to optimize yield rather than purification with batch methods such as ammonium sulfate precipitation (Ward and Swiatek, 2009). These findings are in conformity with the previous works stating that ammonium sulfate saturation level of 60-89% purifies alkaline phosphatase by around 2-5 folds (Bhattacharjee et al., 2004; Mizobutsi, 2012). Subsequent increase in ammonium sulfate saturation level beyond
75% significantly (p<0.05) decreased both the enzyme
yield and purification.
3. CONCLUSION
Components and the pH of the buffer used during the alkaline phosphatase precipitation plays a major role in recovery, because ammonium sulfate precipitation performed using 0.1 M Tris-HCl buffer of pH 8.4 containing 0.1 M MgCl2 and ZnCl2 produced optimum yield and activity in comparision to the 2 M KCl solution of pH 7 both at 0oC. It is more practical to optimize yield
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 341
ISSN 2229-5518
rather than purification at this stage because alkaline phosphatase yield is very poor even when ammonium sulfate saturation of 15% is efficient in enzyme purification. Ammonium sulfate saturation level of 65% efficiently reduces the volume of the homogenate by one third, effectly fractions half of the contaminating proteins away from the precipitate and recovers more than 95% of the alkaline phosphatase activity into the precipitates. REFERENCES
1. McComb, R.B., Bowers, Jr G.N., and Posen, S. (1979)
Alkaline phosphatase, Plenum Press, New York, USA.
2. Hanpongkittikun, A., Siripongvutikorn, S., and Cohen D.L. (1995) ‘Black Tiger Shrimp (Penaeus monodon) Quality Changes during Iced Storage’, ASIAN food Journal, Vol. 10 No. 4, pp.125 – 130.
3. Chuang, N.N. and Yang, B.C. (1990) ‘A comparative
study of alkaline phosphatases among human placenta, bovine milk, hepatopancreases of shrimp Penaeus monodon (Crustacea: Decapoda) and clam Meretrix lusoria (Bivalvia: Veneidae): to obtain an alkaline phosphatase with improved characteristics as a reporter’. Comparative Biochemistry and Physiology B, Vol. 96 No.4, pp. 787 – 789.
4. Nooralabettu, K.P. (2011) Enzyme technology-
Pacemaker of biotechnology. Prentice Hall (India), New
Delhi.
5. Arakawa, T., and Timasheff, S.N. (1985) ‘Theory of
protein solubility’, Methods in Enzymology, Vol. 114 No. 1, pp. 49 − 77.
6. Chiew, Y.C., Kuehner, D., Blanch, H.W. and Prausnitz, J.M. (1995) ‘Molecular thermodynamics for salt-induced protein precipitation’, AIChE Journal, Vol.
41, pp. 2150 − 2159.
7. Hatti-Kaul, R. and Mattiasson, B. (2003) Isolation and
purification of proteins, Marcel Dekker, New York.
8. Wheelwright, S.M. (1991) Protein purification: design
and scale up of downstream processing, John Wiley and Sons,
New York.
9. Nooralabettu, K.P. (2012) Downstream processing-
New horizon in biotechnology, Prentice Hall (India), New
Delhi.
10. Aukhil, I., Joshi. P., Yan, Y. and Erickson, H.P. (1993)
Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins, Journal of Biological Chemistry, Vol. 268, pp. 2542 – 2553.
11. Doonan, S. and Cutler, P. (Eds.) (2004) General
Strategies: Protein Purification Protocols, Humana Press
Inc., Totowa, New Jersey.
12. Mirica, K.A., Lockett, M.R., Snyder, P.W., Shapiro,
N.D., Mack, E.T., Nam, S. and Whitesides, G.M. (2012)
‘Selective precipitation and purification of monovalent
proteins using polyvalent ligands and ammonium
sulfate’, Bioconjugate Chemistry, Vol. 23 No. 2, pp. 293 –
299.
13. Ko, K.Y. and Ahn, D.U. (2007) ‘Preparation of
Immunoglobulin Y from Egg Yolk Using Ammonium
Sulfate Precipitation and Ion Exchange Chromatography’,
Poultry Science, Vol. 86, pp. 400 – 407
14. Moore, P.A. and Kery, V. (2009) ‘High-throughput protein concentration and buffer exchange: comparison of ultrafiltration and ammonium sulfate precipitation’, Methods in Molecular Biology, Vol. 498, pp. 309 – 314.
15. American Chemical Society (ACS) (1999) Lab Guide,
American Chemical Society, Washington, D.C.
16. Puttige, K. and Nooralabettu, K.P. (2011) ‘Alkaline
Phosphatase Activity during Homogenisation of Hepatopancreatic Tissues of Shrimps using Sodium acetate, KCl solution, Tris-HCl and Glycine-NaOH buffer’, International Journal of Scientific and Engineering Research. Vol. 2 No. 10, pp. 1 – 7.
17. Racek, A.A. (1955) ‘Littoral penaeinae from New South Wales and adjacent Queensland waters’, Australian Journal Marine and Freshwater Research, Vol. 6 No. 2, pp.
209 – 241.
18. Puttige, K. and Nooralabettu, K.P. (2012) ‘Effect of
homogenization speed and time on the recovery of alkaline phosphatase from the hepatopancreatic tissues of shrimps’, Food Science and Biotechnology, Vol. 21 No. 2, pp.
461 – 466.
19. Nooralabettu, K.P. and Puttige, K. (2013) ‘Primary
Recovery of Alkaline Phosphatase from the Hepatopancreatic Tissues of Indian white shrimp at Varied Centrifugal Force and Time’. Food Science and Biotechnology, Vol. 22 No. 2, pp. 1 – 7.
20. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and
Randall, R.J. (1951) ‘Protein measurement with the folin phenol reagent’, Journal of Biological Chemistry, Vol. 193, pp. 265 – 275.
21. Bowers Jr., G.N. and McComb, R.B. (1975)
‘Measurement of total alkaline phosphatase activity in
human serum’, Clinical Chemistry, Vol. 21 No. 13, pp.
1988 – 1995.
22. Akita, E.M. and Nakai, S. (1992) ‘Immunoglobulins
from egg yolk: Isolation and purification’, Food Science, Vol. 57, pp. 629 – 634.
23. Bortolato, M., Besson, F. and Roux, B., (1999) ‘Role of Metal Ions on the Secondary and Quaternary Structure of Alkaline Phosphatase from Bovine Intestinal Mucosa’, Proteins, Vol. 37, pp. 310 – 318.
24. Kumar, P., Kumar, A., Kumar, T. and Singh, B.N.
(2012) ‘Purification of Alkaline Phosphatase (ALP), from Streptomyces Sp Js 20, isolated From Mangrove Sediment’, Asian Journal of Experimental Biological Science, Vol. 3 No.
1, pp. 236 – 242.
25. Ward, W.W. and Swiatek, G. (2009) ‘Protein
purification’, Current Analytical Chemistry, Vol. 5 No. 2, pp. 85 –105.
26. Bhattacharjee, S., Das, A.K. and Mandal, S.K. (2004)
‘Isolation and characterization of alkaline phosphatase of
Saccharopolyspora erythraea from fermentation broth of
erythromycin production’, Indian Journal of
Biotechnology, Vol. 3, pp. 558 – 562.
27. Mizobutsi, G.P., Finger, F.L., Ribeiro, R.A.,
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014
ISSN 2229-5518
Puschmann, R., Neves, L.L.M. and Mota, W.F. (2012)
'Effect of pH and temperature on peroxidase and polyphenoloxidase activities of litchi pericarp', Scientia Agricola, Vol. 67 No.2, pp. 213-217.
342
IJSER © 2014
http /lwww <)Ser org



