International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 1
ISSN 2229-5518
Comparative Studies on the Prevalence of Bacterial Population and Physicochemical Analysis in Raw and Pasteurised Milk with special reference to Honey as a Natural Preservative
Dr.V.Karthikeyan.,S.Ramya, Reshma.V.K
Abstract: In this research, fifteen raw milk samples from different areas and ten pasteurized milk samples of various brands were colle cted and compared based on microbiological tests and physicochemical analysis. The milk samples were plated on nutrient agar medium and the isolates were then cultured on different media (EMB Agar, McConkey Agar, Skim Milk Agar). These cultured bacterias were then subjected to confirmative tests. The bacterias isolated were Escheritia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus. The milk samples were further subjected to physico-chemical analysis in which Fat content, Clearance Lactometric Reading, Solid Non Fat and Acidity were calculated. The quality of the milk samples were then analysed by Methylene Blue Reduction Test and Clot on Boiling Test.The isolated bacterias were also tested for their proteolytic and lipolytic property and were also subje cted to catalase tset. Further the effect of honey as a natural preservative of milk was also analysed by studying the dilution of honey at which the maximum zone of inhibition was observed.
———————————————————
S.Ramya- Department of Biotechnology,Karpaga Vinayaga
College of Engineering and Technology
Reshma.V.K- Department of Biotechnology,Karpaga
Milk is an important food with high nutritional value. Raw milk obtained from cow is pasteurised before packing in order to destroy the microorganisms present. Milk is an important growth medium for microorganisms when suitable temperature exists.It gets contaminated and spoiled very easily if handled carelessly and unhygienically The balanced diet milk becomes contaminated with several types of microorganisms which originate from the soil, water etc. Temperature plays a vital role in the spoilage of milk. Microorganisms such as psychotrophs may grow at a temperature of 7°C and they are distributed in diversified habitats such as soil, water, utensils and vegetation.
When the milk is stored under low temperature it gets contaminated and frequently undergoes spoilage due to proteinases and lipases released by the microbes present in the milk i.e psychrotropic bacteria. The presence of these organisms in milk indicate not only unsanitary conditions, but also the yard stick to measure the quality of the products. The psychrotrophs are readily killed by HTST
pasteurization but their extra cellular enzymes are heat stable to varying degrees when sufficient activity may remain to degrade the fats and proteins of milk.
Vinayaga College of Engineering and Technology
When the milk is stored under low temperature it gets contaminated and frequently undergoes spoilage due to proteinases and lipases released by the microbes present in the milk i.e psychrotropic bacteria. The presence of these organisms in milk indicated not only unsanitary conditions, but also the yard stick to measure the quality of the products. The psychrotrophs are readily killed by HTST pasteurization their extra cellular enzymes are heat stable to varying degrees when sufficient activity may remain to degrade the fats and proteins of milk.
Milk is a complex mixture of lipids, carbohydrates and
proteins and many other organic compounds and inorganic salts dissolved in water.
Chemical composition of milk caries due to various factors such as species, breed of chemicals, climate etc
The function of water in milk is to hold the solids of the milk partly in solution and partly in suspension.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 2
ISSN 2229-5518
Milk is a true emulsion of oil in water. Each globule of fat is surrounded by a thin layer which is composed of a complex lipid protein and a small amount of carbohydrate. The lipid portion includes both phospholipids and triglycerides. Milk fat is mixture of glycerides of fatty acids and other lipid materials present in milk are phospholipids.
Milk contains casein and whey proteins. Casein occurs in milk as a colloidal protein- calcium phosphate complex. They are: α Casein: 66%, β Casein: 29%, γ Casein: 5%.
Whey proteins are made up of α-lactalbumin and β- lactoglobulin, serum albumin, enzymes and protease peptones. Whey also contains small amount of lactoferrin and serum transferrin.
The disaccharide lactose is the predominant and distinctive carbohydrate of milk but in addition there is low concentration of monosaccharide including glucose and galactose.
Chlorides, phosphates, citrates, sulphates and bicarbonates of sodium, potassium, calcium and magnesium are present.
by normal pasteurisation procedures and its activity is tested to determine the effectiveness of
pasteurisation.
d) Casein hydrolysis: Casein, the major milk protein, is a macromolecule composed of amino acid subunits
linked together by peptide bonds (CO-NH). Before their assimilation into the cell, proteins must undergo step-by-step degradation into peptones, polypeptides, dipeptides, and ultimately into their building blocks as amino acids. This process is called peptonization or proteolysis, and it is mediated by extracellular enzymes called proteases. The function of these proteases is to cleave the peptide bond CO-NH by
introducing water into the molecule. The reaction then liberates the amino acid.
Raw samples from different areas and pasteurised milk samples of different brands were collected during a period of January-March in the year 2012. The raw milk samples were collected from different areas of Chithamur (Kancheepuram District). The samples were collected whenever required and stored at refrigeration temperature for a period of 2-3 days if needed. The samples were collected in sterilised tubes under aseptic conditions.
9ml of saline in all 6 test tubes and 1ml of milk sample in first test tube then it subjected to serial dilution as 10-1,10-2,10-3,
10-4,10-5 and 1 as control
10g of peptone in one litre of distilled water.Sterilized in the autoclave at 121°C for 15 minutes.)
37°C for 48 hours, following added 5 to 6 drops of methyl red solution into the test and control.Appearance of red colour was indicated, methyl red positive. .
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 3
ISSN 2229-5518
X 100 mm test tube. Small amount of bacterial culture was inoculated into the fluid with the help of a glass rod or plastic loop and the releases of air bubbles were observed and it was compared with the controls.
2.9 Proteolysis: Skim milk agar plates were prepared for inoculation. Bottom of the petridish was divided into two sections. Using sterile technique, single line streak inoculation of each test organism on the agar surface of its appropriately labeled section on agar plate was made. Plates were incubated in an inverted position for 24 – 48 hours at 37oC. Re incubate all negative cultures for an additional 5 days. Milk agar plate culture for the presence or absence of a clear area or zone of proteolysis, surrounding the growth of test organism was examined. Based on the observation, organisms capable of hydrolysing the milk protein casein can be determined.
Three clean and sterilized test tubes were taken. To each of the test tubes 10 ml of milk to be tested was added. Second and third tubes were placed in boiling waterbath for 3 minutes to destroy the natural reducing system of the milk. These two tubes will serve as controls. Then 1 ml of certified methylene blue of 1: 25,000 dilutions to the first tube and 1 ml of tap water to the third tube was added. The milk in the first two tubes will look blue and in the third tube, the milk will
remain white. Incubate all three tubes in waterbath at 37o C
and note the time. The second and the third tubes which serve as controls will not show any colour change. Milk in the second tube will remain blue and milk in the third tube will remain white, while milk in the first tube will gradually become colourless except at the top where the milk is in
contact with air. The second tube as control will indicate when colour change starts in the first tube and the third tube will indicate when the colour change is complete.
S. No | Reduction time | Quality |
1. | More than 8 hours | Excellent |
2. | 6 – 8 hours | Good |
3. | 2 – 6 hours | Fair |
4. | Less than 2 hours | Poor |
10 litre of water.
The fat content was also determined by centrifugation method. Here 10ml of 90% Sulphuric Acid, 10.75ml of milk and 1ml of Amyl Alcohol were taken in a jargon tube and mixed well. This was then subjected to centrifugation at
1200rpm for 5minutes. From that the fat level can be measured.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 4
ISSN 2229-5518
value, ie.. SNF=CLR/4
It was found that artificial honey had no inhibitory effect on the growth of either catalase positive or negative bacteria In this work, artificial honey was diluted with sterilized water at a rate of 5-1 to 5-4. Bacterial isolates from milk sample were streaked on nutrient agar plates. The anti- microbial activity was determined using the agar well diffusion assay method. Using sterile micropipette, 20μl of the diluted artificial honey sample was poured onto each well along with sterilized water as control into a well. The effect of honey was tested against different bacterial isolates from the milk sample. The plates were incubated at 37°C for 24hours. After incubation the plates were examined for evidence of zone of inhibition, which appears as a clear area around the wells. The diameter of such zones of inhibition was measured using a meter ruler.
![]()
![]()
![]()
![]()
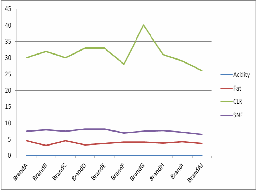
Sample | Acidity | Fat | CLR | SNF |
1 | 0.144 | 4.7 | 30 | 7.5 |
2 | 0.162 | 3.1 | 32 | 8 |
3 | 0.144 | 4.7 | 30 | 7.5 |
4 | 0.081 | 3.4 | 33 | 8.25 |
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 5
ISSN 2229-5518
Acidity : 0.144-0.166
CLR : 20-40
The Clot on Boiling test showed negative result for all samples.
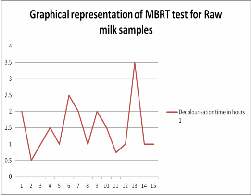
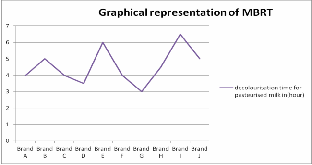
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 6
ISSN 2229-5518
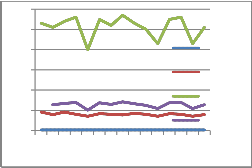
30
25
pasteurised milk samples. The pasteurised samples show greater SNF value.
Further, the pasteurised milk samples were subjected to Phosphatase test in order to test the efficiency of pasteurisation. This showed that the pasteurisation was upto
20 Acidity
15 Fat
10 CLR
5
SNF
0
1 3 5 7 9 11 13 15
The MBRT results are tabulated in Table2&3 for raw and pasteurised milk samples respesctively. In raw milk samples, five samples were fair and ten samples were poor. In pasteurised milk samples, two samples were good and remaining eight were fair enough. Chatterjee et.al (2006) reported excellent quality milk both in raw and pasteurised samples. There are no chances of excellent quality milk in case of raw samples because of the environmental conditions and the cattle feed. In this work, no such excellent quality milk was determined.
With reference to physicochemical analysis, Table-4&5 shows the result for raw and pasteurised milk samples respesctively. In raw milk, the acidity is usually between
0.144-0.166 , CLR is between 20-30. The pasteurised samples show almost the same acidity values, their CLR values lie between 25-40. There is a variation in SNF value for raw and
which may be due to the improper handling and unhygenic conditions.
With reference to microbiological tests done on raw and pasteurised milk samples, the results depicted in Table-III shows the different percentage of bacteria with different characterestics. Four different bacteria were isolated from the samples collected.
Table IV shows the results for the confirmatory test result done to confirm the bacteia. The IMVIC test was done to check the biochemical properties of the isolates.
Further , the antimicrobial activity of artificial honey against the isolates from milk samples showed positive result. N S A Krushna et.al (2005) reported that artificial honey had no inhibitory effect on the microorganisms isolated from the milk samples. In this work, artificial honey depicted a zone of inhibition for Bacillus cereus isolaed from milk which proved that it can be used as a natural preservative for milk instead of chemicals like formaldehyde.
From the results obtained, the following conclusions can be made: Pasteurisation of milk does not alter its physicochemical properties to a great extent with reference to acidity and fat content.The pasteurisation is effective in killing the microbes present in the raw milk samples to a great extent but not completely. The milk processing companies should concentrate on hygeinic conditions during the process. The peservation of milk should be given more attention. They should be preserved natirally instead of chemical preservation which affects the public health. The artificial honey showed a good resistance against the isolate from the milk sample which makes it very effective as a natural preservative.
We are thankful to our Mrs.Meenakshi Annamalai, Director,Karpaga Vinayaga College of Engineering and technology for providing research facility for the successful completion of the project work. The authors would like to acknowledge Prof.T.rangarajulu,Dean, KVCET and V.C.Ravichandiran,Advisor,,KVCET for their Valuable guidance.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 7
ISSN 2229-5518
1. Franciosi E, De Sabbata G, Gardini F, Cavazza A, Poznanaski E (2011) ‘Changes in psychrotrophic microbial population during milk creaming to produce Grana Trentino Cheese’- Food Microbial Feb 28(1).
2. Ammara Hassan, Imran Amjad and Shahid Mahmood (2009) ‘Microbiological and physicochemical analysis of different UHT milks available in market- African Journal of Food Science Vol. 3(4).pp. 100-106.
3. Jeffrey T.LeJeune and Paivi J. Rajala (2009) -Schultz ‘ Unpasteurised Milk: A continued public health threat’- Clinical Infectious Diseases 48:93-100.
4. Laszlo Varga (2007) ‘ Microbiological quality of
commercial dairy products’ – A.Mendez-Vilas(Ed).
5. N S A Krushna, A Kowsalya, S Radha and R B Narayanan (2005) ‘ Honey as a Natural Presrvative of Milk’- Indian Journal of Experimental Biology, Vol.45, pp.459-464.
6. Braun, P. and Fehlhaber, K. (2002). Combined effect of temperature, aw and PH on enzymatic activity of spoilage causing bacteria. Milchwissenschaft. 57(3): 134 – 136.
7. Burdova, O., Barahova, M., Laukova, A., Rozanska, H. and Rola, J.G. (2002). Hygiene of pasteurized milk depending on psychrotrophic microorganisms. Bull. Vet. Inst. Pulawy.
46:325 – 329.
8. Jung, W.( 2002). Bacterial Flora in raw milk views of dairy personnel. DMZ. Lebensittel – Industrie – und – Milchwirtschaft. 123(10): 24 – 30.
9. Prabha, R., Vijaya, G.V., Narayana reddy, K.V. and Bhat, G.S. (2002). Enhancement of functionality of milk proteins by extra cellular proteinases isolated from psychrotrophic organisms in milk. Indian J. Dairy Sci., 55(2): 70 – 73.
10. Sundararaj, T. (2002). Microbiology Laboratory Manual. pp # 218.
11. Pandey, R. and prasad, J.(2001). Influence of treatments of washing of hind quarters and udder on bacterial quality of raw milk. Bioved. 12(1-2):91 – 94.
12. Abdou, S.M., Dawood, A.H., Montasser, E.A. and Ismail, E.E.A. (2001). Evaluation of UHT milk during storage. In: 8th Egyptian conference for Dairy science and Technology held at the International Agriculture centre, Cairo, Egypt, Research papers (I): 149 – 162.
13. Canigova, M. and Benczova, E. (2001). The microflora changes of raw milk during its refrigerated storage. Acta- Fytotechnica-et-zootechnica. 4(4): 104 – 106.
14. Dawood, A.H., Abdou, S.M., Montasser, E.A. and Ismail, E.A. (2001). Microbiological evaluation of Egyptian ulta-high temperature (UHT) milk. In: 8th Egyptian conference for
Dairy science and Technology held at the International
Agriculture centre, Cairo, Egypt, Research paper (I): 133 –
147.
15. Geetha, R. and Prasad, V. (2001). Studies on the performance of cultures of lactic acid bacteria in lactose hydrolysed buffalow skim milk. Cheiron. 30(3-4): 81 – 84.
16. Kirin, S. (2001). The effect of cooling procedure on the characteristics and quality of raw milk. Mijekarstvo. 51(2):
151 – 159.
17. Mehanna, N.M., Moussa, M.A.M. and Ahwall, R. (2001). Improving quality of milk during its cold storage. Egyptian J. Dairy sci.,. 29(1): 9 – 18.
18. Carvalho, F. and Luchese, R.H. (2000). Profile of raw milk spoilage microbiota important for UHT milk processing. Anais do XVII congresso Nacional de Laticinios. Perspectivas Advances em Laticinios. 54(318): 188 – 195.
19. Kolakowski, P., Reps. A. and Fetlinski. A. (2000). Microbial quality and some physicochemical properties of high pressure – processed cow milk. Polish journal of food and Nutrition Sciences. 9(4): 19 – 26.
20. Lira, F.C., Oria, M., Hayes, K.D. and Nielsen, S.S. (2000). Effect of Psychrotrophic bacteria and of an isolated protease from Pseudomonas fluorescens M315 on the plasmin system of fresh milk. J. Dairy Sci., 83: 2190 – 2199.
21. Mohran, M.A., Fahmy, M.A., Elhoda Hanary, N. and Nanis, H.G. (2000). Influence of lactic acid bacteria starters on the proteolytic activity of thermoduric bacteria isolated from milk. Assiut Journal of Agricultural sciences. 31(1): 261 –
269.
22. Vyletelova, M., Urbanova, E. and Benda, P. (2000). The incidence of psychrotrophic microorganisms in raw cow milk and the effect of proteolytic and lipolytic enzymes on technological traits of milk. Vyzkum-V-Chovu – Skotu. 42(3): 1
– 10.
23. Vyletelova, M. Hanus, O. Urbanova, E. and Kopunecz, P. (2000). The occurrence and identification of psychrotrophic bacteria with proteolytic and lipolytic activity in bulk milk samples on storage in primary production conditions. czech Journal of Animal Science. 45(8): 373 – 383.
24. Wiedmann, M., Weilmeier, D., Dineen, S., Ralyea, R. and Boor, K.J. (2000). Molecular and phenotypic characterization of Pseudomonas Isolated from milk. Applied and Environmental Microbiology. pp # 2085 – 2095.
25. Cappuccino, J.G. and sherman, N. (1999). Microbiology A Laboratory manual. Benjamin / Cummings publishing company, New York. pp # 477.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 6, June-2012 8
ISSN 2229-5518
26. Jayarao, B.M. and Wang, L. (1999). A study on the prevalence of Gram negative bacteria in Bulk tank milk. J. Dairy Sci., 82:2620 – 2624.
27. Meisel, H. and Backelmann, W. (1999). Bioactive peptides encrypted in milk proteins, proteolytic activation and thropho-functional properties. AntonievanLeeuwenhoek. 76(1-
4): 207 – 15.
28. Santos, E.de.S., Carvalho, Ep.de. and Abrev, L.R. de. (1999). Psychrotrophic bacteria consequences of their presence in milk and cheeses. Boletim – da – Sociedade – Braisileria – de – ciencia – e – Techno – logia – de – Alimentos. 33(2): 129 – 138.
29. Srilakshmi, B. (1999). Food science. New age
International private limited publishers, Delhi. pp # 521.
30. Patke, D. and Dey, S. (1998). Proteolytic activity from a thermophilic Streptomyces megasporus strain SDP4. Letters in applied microbiology. 26: 171 – 174.
31. Ibrahim, S.A. (1998). Agglutination Behavior of Mesophilic starter cultures as a function of proteolysis. J. Food prot., 61(7): 855 – 858.
32. Griswold, K.E. and Mackie, R.I. (1997). Degradation of protein utilization of the Hydrolytic products by a predominant Ruminol bacterium, Prevotella ruminicola B14. J. Dairy Sci.,
80:167 – 175.
33. Huang – chienJung. and Huang, C.J. (1996). Effect of proteolytic products produced by other lactic acid bacteria in milk on the growth of Bifidobacterium longum. Journal of the chinese society of Animal science. 25(2): 221 – 230.
34. Aslam, M. and Hurley, W.L. (1996). Proteases in Milk.
Illinois Dairy Report Home Page. 1 – 3
35. Celestino, E.L., Iyer, M. and Roginski, H. (1996). The effects of refrigerated storage on the quality of raw milk. Australian J. Dairy Tech., 51(2): 59 – 63.
36. Stancheva, N. and Petrova, N. (1995). Technological conditions for cleaning milking machines for ewes using a combined alkaline detergent, and their influence on the microbiological quality and biochemical properties of milk. Zhivotnovdhi – Nauki. 32(5 – 8): 325 – 328.
IJSER © 2012