
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1119
ISSN 2229-5518
Anita A. Pandit, Ramdas A. Pawar, Dnyaneshwar R. Shinde
Abstract—Colloidal MnO 2 has been synthesized and characterized by chemical and spectroscopic methods. Chemical analysis showed that synthesized MnO 2 is 99.21% pure and X-ray spectroscopic analysis indicated that it is amorphous in nature. Scanninig electron microscopy observation revealed that amorphous MnO 2 consists of fine-particles of rougly spherical in shape with the average particle size
210 nm. Degradation of the dyes from aqueous solution is accounted in terms decolorization of the dye solution. Preliminary experiments showed that synthesized MnO 2 is capable of decolorize selected azo dyes efficiently from aqueous solution. Then further experiments were carried out to optimize reaction conditions. pH dependent degradation of these dyes showed that pH below five support the decolorization of both the azo dyes. Kinetics of degradation of dyes showed that time 60 to 75 minutes is sufficient for the complete decolorization of both azo dyes from aqueous solution. Effect of temperature was investigated and it has been observed that rate of degradation of azo dyes increases with increase in temperature of the dye solution.
Index Terms— azo dyes, degradation, colloidal MnO2 , decolorization, catalyst, methyl orange, methyl red.
—————————— ——————————
ZO dyes are characterized by –N=N– and chromophoric group attached to nitrogen atoms which are responsible
for the characteristic colours of the azo dyes [1]. More than
10,000 dyes are used in textile, leather, paper, food, petroleum,
etc. industries, and from them about 50% dyes are azo dyes
[2]. From the total amount of dyes used in the textile indus-
tries 10 to 20% dyes are released into effluent either in solution
or suspended form [3]. It was estimated that about 280,000
tonnes of dyes are discharged every year into effluent world-
wide [4]. Disposal of azo dyes in natural water sources cause many issues regarding water pollution. Such as aerobic deg- radation of azo dyes in water leads to the formation of aro- matic amines which are more toxic in nature than original dye
[5]. Chemical oxygen demand (COD) of azo dyes is very high as compared to biological oxygen demand (BOD) which indi- cates that they are not easily biodegradable. Moreover azo dyes and their degradation product are toxic, mutagenic, and carcinogenic in nature [6]. Dyes possess high absorbance. Hence even though dyes are present in minute quantity they are clearly visible in water. This property of dyes limits pass- ing of the light through water and directly affects life of phyto- planktons by showing adverse effect on photosynthesis pro- cess [7]. Therefore it is of prime importance to remove dyes from industrial effluent. For removal of organic compounds biological methods are used however, these methods are not much more effective for removal of dyes due to resistance of dyes towards biodegradation [5, 8, 9]. Many dye stuffs are designed so that they are resistance to degrade by light as well as by microorganism. Today advanced oxidation process are used which are proven to be more effective and able to de-
————————————————
• Corresponding Author: Dnyaneshwar R. Shinde working as Associate Profes- sor at Chemistry Department, Prof. Ramkrishna More College, Akurdi, Pune –
411044. (Pune University, India) E-mail: drshinde1970@yahoo.com
• Co-Author: Anita A. pandit is currently pursuing Ph. D. degree from JJT University, ZunZun, Rajasthan (India).
• Co-Author: Ramdas A. pawar working as Associate Professor at Chemistry
Department, PDEA’s Baburaoji Gholap College, Sangavi, Pune-411027
grade dye completely from waste water [10, 11, 12]. However, these processes has drawback such as high enegy and chemi- cal requirement, formation of side products, etc. [13]. In ad- vanced oxidation process like Fenton’s oxidation process for- mation of Fe(OH)3 takes place while in persulfate method formation of NH4 SO4 takes place as a side products. Side products are not desired into treated effluents hence formation of side products increases the load on the treatment process. In present investigation we are proposing simple and cost effec- tive catalytic method for degradation of two azo dyes by using manganese dioxide as a catalyst. There are very few reports on manganese dioxide based degradation of azo dyes hence we have tried to explore use of MnO2 as a catalyst for dye degra- dation. Methyl orange and methyl red are selected as model azo dyes in our study.
2.1 Preparation of Catalyst:
Colloidal MnO2 was prepared by the reaction between
MnSO4 and KMnO4 under neutral pH. To definite volume of
0.1M MnSO4 solution 0.1M KMnO4 solution was added drop wise with constant stirring till supernatant solution acquire
faint pink color. Then resulted brown colored solid was sepa- rated by filtration using Whatman filter paper No-41 under vacuum. Precipitate was washed on filter paper with distilled water till it become free from sulfate ions. Brown colored solid was dried in an oven at 110°C for 2 hr. and used as catalyst.
2.2 Characterization of Colloidal MnO2 catalyst:
Weighed quantity of catalyst was dissolved in 1:2 conc. HCl: HNO3 and Mn content in solution was determined by Volhard’s method [14]. SEM, XRD spectrum of synthesized MnO2 was recorded.
2.3 Primary Experiment:
Solutions of dyes methyl red (MR) and methyl orange (MO) were prepared at the concentration 1×10-4 M. Both dyes are of high purity and purchased form Sigma-Aldrich Chemi- cal Company. To 100 ml dye solution 200 mg synthesized
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1120
ISSN 2229-5518
MnO2 was added and resulted suspension was stirred at 200 rpm on magnetic stirrer for 90 minutes then suspension was centrifuged and analyzed spectrophotometry for dye content in the solution. The residue of MnO2 in the centrifuge tube was washed with water, then stirred with 15 ml 2M CH3 COOH for 10 minutes and finally centrifuged. Superna- tant was collected and analysed by spectrophotometry for dye content.
2.4 pH Dependent Degradation of Dyes:
pH of 100 ml dye solution was adjusted to desirable value
±0.1 by 1 or 0.1N H2 SO4 , to it 100 mg MnO2 was added and stirred at 200 rpm for 60 minutes. After 60 minutes suspension was filtered and quantity of dye remaining was determined by
spectrophotometric method. This experiment was performed at different pH of dye solution from pH 2 to 7 at the interval of one unit.
2.5 Time Required for Complete Decolorization:
pH of 200 ml effluent was adjusted to requisite value (pH at which degradation of dye was observed maximum) and 200 mg MnO2 was added to it. Resulted suspension was stirred at
200 rpm on magnetic stirrer till complete decolorization of dye solution take place. During experiment 5 ml suspension was withdrawn at definite time interval, centrifuged and analyzed for dye content by the spectrophotometric method.
2.6 Recovery of Catalyst:
To the treated dye solution 1M KMnO4 was added till solution shows slightly pink color. This solution was centrifuged to separate solid MnO2 . Brown colored solid MnO2 was dried and reused for next catalytic cycle.
2.7 Determination of Dye Concentration
Methyl orange has λmax 490 nm methyl red has λmax 510 nm at pH = 4. Absorbance of dye solutions was determined at these wavelengths and dye concentration was calculated by compairing absorbance of sample with standard.
Cstd
Csam = Astd × Asam × dilution factor
Where, CRsamR – dye conc. in sample solution, CRstdR-conc. of dye in standard solution, ARstdR-absorbance of standard solution of the dye, ARsamR-absorbance of sample of the dye.
3.1 Synthesis of MnOR2
Reaction between MnSOR4R and KMnOR4R take place at the neutral pH and room temperature to form MnOR2R which can be represented as follows. This reaction is a basic reaction of Volhard’s method for the analysis Mn(II) by using standard KMnOR4R as an oxidizing agent [13].
3MnSO4 + 2KMnO4 +2H2O → 5MnO2 + 2H2SO4 + K2SO4
In this reaction Mn(II) from MnSOR4R is oxidized to MnOR2R while Mn(VII) from KMnOR4R is reduced to MnOR2R i.e. redox reaction take place between Mn(II) and Mn(VII) to form Mn(IV) i.e. MnOR2R. MnOR2R is water insoluble and appears as colloidal brown-black colored solid product in solution.
3.2 Properties of MnOR2
Chemical analysis of dried colloidal MnOR2R showed that it contains 99.21% of MnOR2R i.e. synthesized MnOR2R is highly pure. Scanning electron microscopy observation revealed that the amorphous MnOR2R fine-particles were roughly spherical, uniformly distributed, and not highly agglomerated (fig. 1 a) and surface is porous in nature (fig.1 b). It is found that the average partical size of MnOR2R powder is 210 nm.

XRD spectrum (fig. 2) do not showed any characteristic peak indicating that synthesized MnOR2R is non-crystalline in nature.

Fig.1(a): SEM photograph of synthesized MnO2
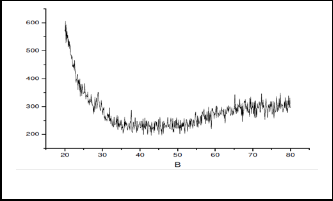
Fig.1(b): SEM photograph of synthesized MnO2
Fig.2 - XRD spectra of synthesized MnO2
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1121
ISSN 2229-5518
3.3 Primary Experiment
Primary experiment was performed to evaluate ability of the synthesized MnOR2R to degrade / decolorize MO and MR dyes from the aqueous solution. Results of primary experi- ment showed that on treatment with MnOR2 Rabout 91% MO and 48% MR solution were decolorized indicating that MnOR2R has ability to decolorize these dyes under ambient condition. Decolorization by metal oxide may occur due to the surface adsorption or by catalytic degradation. MnOR2R which was used for dye colorization was treated with 2M CHR3RCOOH and in the process dye is not released in the CHR3RCOOH solution, in- dicating that contact between MnOR2R and dye resulted into degradation of dye. Our observation is also supported by Jiantuan and Jiuhui [3] and Clarke et. al. [14]. Therefore, fur- ther experiments were carried out to optimize the reaction conditions.
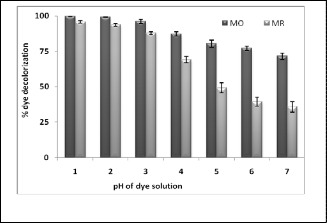
3.4 Effect of pH
This experiment was performed to investigate best pH for the degradation of MO and MR by colloidal MnOR2R as a catalyst. Results depicted in (fig. 3) clearly indicate that rate of degradation of azo dyes is governed by pH of the dye solu- tion. In case MR percent degradation goes on continuously increasing with decrease in pH of the dye solution and below pH 4 appreciable degradation was observed that at pH 5 to 7. In comparison to MR, effect of pH is less marked on degrada- tion of MO and appreciably high degradation of MO was ob- served at all the pH used in experiment (above 71.52%). De- pending on results of this in further experiments different pH are used for both dye, these are 5 for MO and 3 for MR. Clarke et. al. [14] also has reported similar results on oxidation of acid orange-7 by manganese oxide a catalyst. They observed ap- preciable high oxidation of an azo dye acid orange-7 blow pH
5. At pH below 5, H+ ion concentration in solution is suffi- ciently high to cause protonation of MnOR2R at surface which generate active chemical species like MnOH2+, MnOH, MnO- etc. In the process electron transfer take place from dye to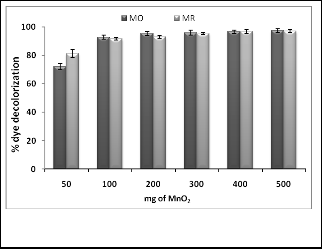
tity of MnOR2R that should be used for complete degradation of particular dye. The results depicted in (fig. 4) clearly point out that the quantity of dye degraded goes on increasing with in- crease in quantity of MnOR2R however percent degradation as- sume nearly constant value above 200 mg MnOR2R per 100 ml dye solution. As such from 100 to 500 mg quantity of MnOR2R significant difference was not observed in quantity of dye de- graded. Thus quantity such as 1mg per ml is sufficient to carry out degradation of dye from water solution within 60 to 90 minutes. It is well known fact that rate of a chemical reaction increases with increase in quantity of catalyst in a reaction and at particular value it assumes constant value. Similar results were observed in our experiment.
Fig.4. Effect of catalyst dose on percent of dye decolorization
3.6 Kinetics of Dye Degradation
This experiment was performed to investigate time required for complete degradation of the dye from definite volume with fixed quantity of MnOR2R. The results summarized in fig. 5 and 6 point out that for both dyes initial rate of degradation is veyhigh which goes on decreasing logarithmically with time. In case of MO, in first 10 minutes about 76% degradation has
MnOR2R [3]. High H
ion concentration solution also help to
taken place while for about 100% degradation 50 minutes
form side product and removal of sideproduct from surface of
MnOR2R [15].
were required while for MR about 52% degradation of dye
had taken place in first 10 minutes and for complete degrada-
tion about 75 minutes were required. Initially high rate of deg-
radation was observed which is due to high initial concentra- tion of dye in the solution.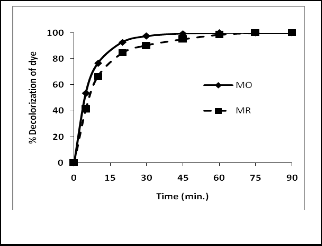
Fig.3. Effect of pH on percent of dye decolorization
3.5 Effect of Catalyst Dose
This experiment was performed to know minimum quan-
IJSER © 2 http://www.ijser.org
Fig.5. Kinetics of dye decolorization
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1122
ISSN 2229-5518
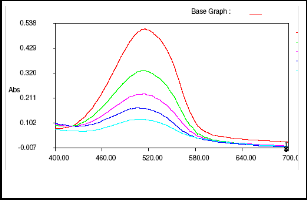
With time dye concentration goes on decreasing in solution hence rate of reaction also goes on decreasing. Thus, after 30 minutes time of the reaction, rate of change of concentration of the dye with time is very less which results into long time for complete degradation of the dye from solution. Comparison of results of kinetics of degradation of two dyes demonstrates that for 100% degradation MR required signficantly higher time than MO. From these observations we can conclude that MO is more prone towards degradation by MnOR2R than MR. The time required for degradation MO and MR is comparable or even low than by other reported [13, 16].
Fig.6. Visible spectra of MR at different time interval
TABLE -1
Percent decolorization of dyes at dfferent temperatures
Temp (°C) | % Decolorization | |
Temp (°C) | MR | MO |
26 | 91.72±1.03 | 98.90±1.49 |
35 | 98.17±0.94 | 100 |
50 | 100 | 100 |
observed effect is not too distinct at temperature 26, 35 and 50
°C. At 50 °C 100 % degradation was observed for both dyes in
less than 45 minutes. MR showed 91.72±1.03% degradation at
room temperature while 98.17±0.94% degradation at 35 °C within 60 minutes time. Similar pattern of result was observed for MO at room temperature and at 35 °C. Temperature de- creases energy of activation and there by increases rate of deg- radation reaction of dyes.
Colloidal MnOR2R can be used as heterogeneous catalyst for degradation of the azo dyes and it is possible to remove azo dyes completely from aqueous solution by degradation pro- cess. Process is simple and costeffective as catalyst is regener- ated and reused. However, further work must be carried out to so that method can be adopted for large scale treatment of effluent containing varietry of the azo dyes.
[1] C. L. Hsueh, Y. H. Hung, C. C. Wang, S. Chen, “Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system”. Chemo- sphere, 58, 1409, 2005.
[2] R. Kannan, S.G. Peer A.. Obadiah, S. Vasanthkumar, “MnOR2R supported
POM–a novel nanocomposite or dye degradation”. Digest Journal of Nano- materials and Biostructures, Vol. 6 (2), p. 829 – 835, 2011.
[3] G. Jiantuan, Q. Jiuhui, “Ultasonic irradiation enhanced degradation of azo dye on MnOR2R”. Applied Catalysis B: Environmental, 47, p.133-140, 2004.
[4] R. Mass, S. Chaudhari, “Adsorption and biological decolorization of azo dye
Reactive Red -2 in semi continous anaerobic reactors.” Process Biochem. 40, p.
699-705, 2005.
[5] K.T. Chug, G.E. Fulk, M. Egan, “Reduction of azo dyes by intestinal anaer- obes”. Applied Environmental Microbiol. 35, p. 558-562, 1978.
[6] Garner and N. Madhusudhana, “Catalytic degradation of ccoralene Dark Red
2B azo dye by using calcium zincate nanoparticles”. International Journal of
Chemical Engineering and Applications, vol. 2 (4), p. 294-297, 2011.
[7] F. Cicek, A. Ozer, “Low cost removal of reactive dyes using wheat bran”.
Journal of Hazard Mater., 146, p. 408-416, 2007.
[8] U. Pagga, D. Brown, “The degradation of dye stuff part-II: beheviour of dye- stuff in aerobic bidegradation test.” Chemosphere, 15, p. 479-491, 1986.
[9] O. Tunay, I Kabdasli, G. Eremektar, D. Orhon, “Colour removal from textile waste water”. Water Sci. Technol., 34, p. 9-11, 1996.
[10] S. Y. Yang, Y. Y. Chen, H. Z. Xu, P. Wang, Y. H. Liu, M.D. Wang, “A novel advanced oxidation technology: Activated persulphate”. Pprogress in Chem- istry, 20(9), p. 1433-1438, 2008.
[11] H.Y. Shu and M.C. Chang, “Decolorization effects of six azo dyes by OR3R ,
UV/OR3R and UV/HR2R OR2R processes”. Dyes and Pigments, vol. 65(1), p. 25-31.
2005.
[12] R. B. M. Bergamini, E. B. Azevedo and L. R. R. D. Araujo, “Hetergeneous photocatalytic degradation of reactive dyes in aqueous TiOR2R suspension: de- colorization kinetics”. Chemical Engineering Journal, vol. 149, vol. 1(3), 215-
220, 2009.
[13] Y.P. Chen, S.Y. Liu, H.Q. Yu, H. Yin, Q.R. Li, “Radiation-induced degradation of methyl orange in aqueous solutions”. Chemosphere, 72, p. 532–536, 2008.
[14] A.I. Vogel, “Textbook of Inorganic Quantitative Analysis”. 3rd Ed., ELBS, pp
296-297, 1964.
[15] C.E. Clarke, F. Kielar, H.M. Talbot, and K. L. Johnson, “Oxidative decoloriza- tion of Acid Azo dyes by a Mn oxide containing waste”. Environ. Sci. Tech- nol., 44, 1116-1122, 2010.
[16] J.Q. Chen, D. Wang, M. Zhu, C.J. Gao, “Study on degradation of methyl or- ange using pelagite as photocatalyst”. Journal of Hazardous Materials,
B-138, p. 182–186, 2006.
IJSER © 2013 http://www.ijser.org