Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h, Vo lume 2, Issue 12, Marc h-2012 1
ISS N 2229-5518
Coconut leaves as a low cost adsorbent for the removal of Nickel from Electroplating effluents
Rudre Gowda 1, A.G.Nataraj2 and N.Manamohan Rao3
Abs tract— This s tudy wa s focuse d on coconut lea ves as a n a lte rna tive a dsorbe nt for the remova l of Ni(I I ) from was te wa ter. Ba tch e xperime nts we re conducte d a t room te mpera ture 27 0C to de te rmine the factors a ffecting a ds orption of Ni(I I ). The a ds orption process is a ffecte d by va rious pa rame ters s uch as contact time , s olution pH, a ds orbent dose a nd initia l conce ntra tion. The ma ximum remova l e fficie ncy of Ni(I I ) was 93.18% for 2.0g/50ml of coconut lea ves a t pH 8.0 in optimum time of 4 hours . The e xpe rimenta l da ta wa s tes te d us ing La ngmuir a nd Fre undlich equa tions . The da ta fitte d we ll to both La ngmuir a nd Fre undlich is o therms . The a dsorption kine tics we re bes t describe d by the pse udo second orde r mode l. The cos t of remova l is e xpe cte d to be quite low, as the a ds orbe nt is chea p a nd eas ily a va ilable in la rge qua ntities . The present s tudy s howe d tha t coconut leaves was ca pable of removing Ni(I I ) from a queous solution.
Key words: Ads orption, Coconut lea ves, Nicke l(II ).
—————————— ——————————
In India, ther e ar e mor e than 50,000 lar ge, medium, and small scale electr oplating units mostly scatter ed in urban ar eas[2] and ther e ar e about 79 electr oplating industries located in Karnataka state, out of which 71 industr ies ar e in and ar ound Bangalor e city only[3]. The water consumption is less in electr oplating industries compar ed to other industr ies, and the effluent is mor e toxic than other wastes. These industr ies pr oduce toxic hazar dous waste containing heavy metals approximately 78,000kg/annum which adver sely effect envir onment , especially humans, animals, plants, and aquatic life [3].
When waste water containing heavy metals flows on the sur face of the gr ound, it looses fertility and the waste water containing nickel, or iginates pr imar ily fr om metal industr ies, particular ly dur ing plating operations. Occurr ence of dermatitis in some w or kers engaged in electr oplating, polishing paints and pigments may be attr ibuted to nickel poisoning.
Heavy metal toxicity can r esult in damage or r educed mental and centr al nervous function, lower ener gy levels and damage to blood composition, lungs, kidneys, liver and or gans[4].
-----------------------------------------------------------------------------
1. Assistant Pr ofessor of Civil Engineer ing, Bangalor e Institute
of Technology. K.R.Road, Bangalor e – 560004
2. Pr ofessor and Head of Civil Engineer ing, Bangalor e
Institute of Technology. K.R.Road, Bangalor e –560004
3. Former Pr ofessor of Envir onmental Engineer ing, Bangalor e
University.
Heavy metals can pose health hazar ds, if their concentration exceeds allowable limits[5]. Ther efor e, befor e waste water flow s thr ough waterbodies or land, it should be tr eated.
Effluents fr om industrial pr ocesses such as electr oplating, mining, nuclear power operation, battery man ufactur ing, dye and pigment have been identified to contain high level of heavy metals, such as Cr (III), Cr (VI), Zn, Cd, Cu, Ni, Hg and Pb [6].
The methods adopted ar e ion exchange, electr ochemical r eduction, evapor ation, solvent extraction, r ever se osmosis,
chemical pr ecipitation, membrane filtration, and
adsor ption[7].
Many r esear cher s have identified the low cost adsorbent like saw dust [8], r ice husk [9], coir pith[10], coconut shell, waste tea pow der, coconut husk[11], sugar cane bagasse [12] and others.
Even though the industr ies ar e not keen to adopt these adsorbents, all industries ar e adopting chemical pr ocesses only, due to difficulty in disposing of adsorbent mater ials after use.
Ther efor e, it is important to identify an adsorbent material like coconut leaves for r emoval of heavy metals in electr oplating industr ial effluents which is having advantages of r emoval of pollutants fr om effluents effectively and do not have much adverse impact on environment when disposed after tr eatment. The main obj ective of the exper iment is to r emove the Ni(II) fr om waste water using low cost and locally available adsorbent mater ial and alter native tr eatment method.
IJSER © 201 2
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h Vo lume 2, Issue 12, Marc h-2012 2
ISSN 2229-5518
‘fr onds’, which ar e generally 4 to 6 meter s in length and 1.5 to
2 meters in w idth. Leaves have a str ong r achis to which the leaflets ar e linear-lanceolate. Canopy of coconut (cr own) consists of 28 to 36 fr onds at the tip of the stem arranged in cir cular shapes. Gener ally, one frond is added to the canopy every month and one fr ond is abscessed fr om the stem.
(Tested in B.I.T. labor atory)

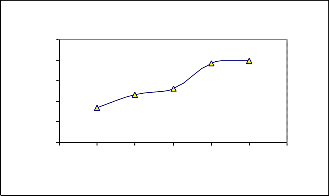
Effect of contact time on Ni(II) removal using
Coconut leaves
50
40
30
20
(Tested in Civil Aid, Bangalor e- 2011)
Preparation of adsorbent: Coconut leaves w er e obtained fr om Mandya distr ict. These leaves was washed with distilled water, dr ied, and power ed. These power ed leaves w er e sieved with IS sieves (1000-600µ). Sieved samples was washed w ith distilled water and dr ied at room temper atur e 270 C for 24 hours, and then samples w as dr ied in hot air oven at a temperatur e 420 C for 8 hours and later cooled in r oom temperatur e and pr eserved in air tight plastic container.
A stock solution containing 1000mg/l of Ni (II) was pr epar ed by dissolving the pur e nickel metal in 1:1 hydrochlor ic acid solution and then diluting the same up to 1000ml in a volumetric flask with double distilled water.
10
0
0 60 120 180 240 300 360
Contact Tim e in m inute s
C0 =26.81m g/l, V=50m l
The r esults for the effect of contact time on adsorption of Nickel (II) r emoval ar e shown in Fig(1). 0.2 to 2.0g of adsorbent was used for this exper iment in contact time 60 m i- nutes to 300 minutes. The per centage r emoval efficiency of nickel (II) ions incr eases w ith incr ease in contact time. The equilibr ium contact time was established at 240minutes as shown in Fig(1) w hich was used as optium contact time for further tests.
At the initial stage, the rate of r emoval of Ni(II) was higher due to availabilty of mor e number of active sites on the sur face of the adsorbent and became slower after 240minutes, due to decr eased or lesser number of active sites. Similar observation has been r eported in liter atur e [13].
IJSER © 201 2
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h Vo lume 2, Issue 12, Marc h-2012 3
ISSN 2229-5518
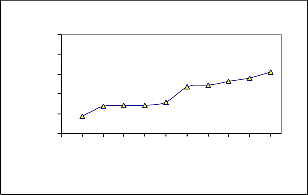
Effect of adsorbent dosage of Ni(II) on Coconut leaves
100
96
92
88
84
80
at pH 8 was maximum and at pH 2 was minimum and slightly decr eases at pH 10-12 as shown in fig (3). At low pH values, the adsorption per centage is low due to the positive char ge density (pr otons) on the sur face sites, r esulting in the electr ostatic r epulsion betw een the Ni(II) ions and edges gr oups wit h positive char ges (Si-OH2+ or Ca-OH2+ ) on the sur face. Electr ostatic r epulsion decr eases with incr easing pH because of r eduction of positive char ges density on the sor ption edges, thus r esulting in an incr ease in Ni(II) ion adsor ption on the sur face.
0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 2
Weight of adsorbent in grams
C0 =26.81mg/l, V=50ml, pH 8.0, t=240min
The r esults for the effect of w eight of adsorbents on adsor ption of Nickel (II) r emoval using Coconut leaves ar e shown in fig (2). 0.2 to 2.0g of Coconut leaves w er e used in this experiment for r emoval of Ni (II).
The per centage r emoval adsor ption incr eased as the adsorbent
dosage is incr eased. This shows that by incr easing the adsorbent dosage the efficiency of coconut leaves incr eases, while adsorption density decr eases with incr ease in adsorbent dosage. The decr ease in adsor ption density may be due to the fact that some adsorption site has r emained unsaturated dur ing the adsor ption process wher eas; the number of sites for adsor ption incr eased by incr easing the adsor ption dosage and that r esulted in the incr ease of r emoval efficiency. Similar observations ar e r epor ted in liter atur e [4], [6], [14].
In an Alkaline medium, the sur face of coconut leaves becomes negatively char ged. Refering to Fig(3), the maximum adsor ption of Ni(II) ion occurr ed at pH 8. At pH values higher than 8, Ni(II) pr ecipitated as hydr oxide which decr eased the rate of adsorption and subsequently the per cent r emoval of Ni(II) ions. Similar observation was r eported in literatur e [15].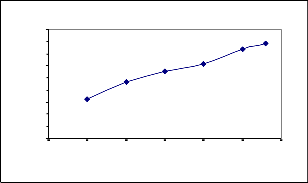
Effect of Initial concentration on Ni(II) removal using
Coconut leaves
90
80
70
60
50
40
30
20
10
0
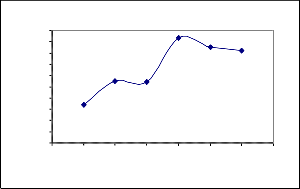
Effect of pH on Ni(II) removal using Coconut leaves
0 5 10 15 20 25 30
Efflue nt Conce ntration in m g/l
Co=26.81m g/l, V=50m l, t=240m in
100
90
80
70
60
50
40
30
20
10
0
0 2 4 6 8 10 12 14 pH
Co=26.81m g/l, V=50m l, t=240m in
The exper iment was carr ied out over initial concentr ations
5mg/l to 26.81mg/l per 50ml of solution and at a contact time of 1-5 hours w ith 0.2 to 2.0g of coconut leaves with a pH of
2.42. The minimum and maximum r emoval efficiency of nickel is 32.702% and 78.41% r espectively as shown in Fig(4).
The experimental r esults of adsorptions of nickel ion on the electr oplating effluent at var ious concentr ation (5, 10, 15, 20 and 26.81mg/l) with contact time of 4hours as shown in Fig(4).It r evelead the adsor ption capacity incr eased w ith incr ease in the Ni(II) concentration. This was due to higher
The adsor ption capacity of adsorbent as function of effluents pH was 2.42. The exper iment was conducted over pH range of
2 to 12. pH is one of the most important parameters contr olling the adsor ption pr ocess. The effect of pH of the solution on the adsorption of nickel ion on coconut leaves was determined. The pH of solution was contr olled by the addition of 0.1M of HCL or 0.1M of NaOH.The uptake of the nickel ions
pr obabilities of collusion between metal ion and adsorbent.
Similar observation was r eported in literatur e [16].
IJSER © 201 2
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h Vo lume 2, Issue 12, Marc h-2012 4
ISSN 2229-5518
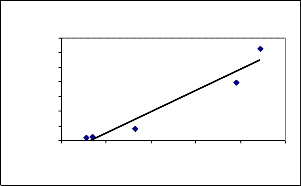
7000
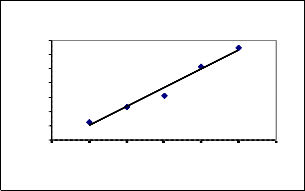
Ps e udo s e cond orde r m ode l for Ni re m oval us ing Coconut le ave s
140
6000
5000
4000
3000
2000
1000
0
y = 144.9x - 956.43
R2 = 0.9403
0 10 20 30 40 50
Co=26.81m g/l, V=50m l, pH 8.0
120
100
80
60
40
20
0
y = 0.4438x - 7.0323
R2 = 0.9759
0 60 120 180 240 300 360
Tim e t in m ins
Conce ntration=26.81m g/l, V=50m l, pH 8.0
0
-0.5
-1
-1.5
-2
-2.5
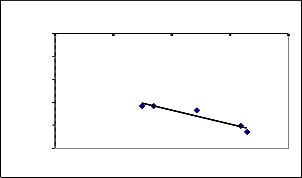
Freundlich isotherm for Ni(II) removal using Coconut leaves
0 0.5 1 1.5 2
y = -0.601x - 1.0729
R2 = 0.9002
log Ce
V=50m l, Co=26.81m g/l, pH=8.0
The equilibrium adsor ption study of Nickel(II) was done to study the kinetics of the r eaction involved. Using experimental data, the pseudo fir st or der (figur e not shown) and secon d or der model ar e drawn as shown in Fig (7).
It was found that mean value of Regr ession co-efficient (R2 ) is
0.3477 for pseudo first or der equation and 0.92 28 for pseudo
second order equation. The data confirms the pseudo second
or der r eaction and r esults ar e shown in table (4). Similar ob servations r eported in literatur e [4].
Several equilibrium models have been developed to descr ibe adsor ption isotherm r elationships. The adsor ption of Ni(II)ions was carr ied out at differ ent initial concentr ations ranging fr om 5 to 26.81 mg/l with contact time 4 hour s. The data obtained w er e analyzed w ith the Langmuir and Fr eundlich isotherm equations as shown in Fig (5 and 6).
The meanvalues of the r egr ession co-efficient (R2 ) is found to be 0.9383 and 0.8242 in Langmuir and Fr eundlich isotherm equations r espectively. The r esult shows that the experimental data best suits Langmuir isother m than the Fr eundlich isotherm. The r esults ar e shown in the table (3).
The adsorption behaviour of Nickel on coconut leaves was investigated in batch equilibrium adsor ption. The adsor ption was found to be drastically dependent on pH, adsorbent dosage and contact time. The optimum pH for Ni ion was found to be 8. The r ate of Ni adsor ption was rapid using coconut leaves with equilibr ium contact time of 4hours.
IJSER © 201 2
Inte rnatio nal Jo urnal o f Sc ie ntific & Eng inee ring Re se arc h Vo lume 2, Issue 12, Marc h-2012 5
ISSN 2229-5518
Isotherm analysis of the data show ed that, the adsor ption patter n of Ni(II) follow ed by the Fr eundlich isotherm equations. The maximum adsorbent dosage was 2.0g/50ml, used for tr eatment of electr oplating effluent. Kinetics data is confirmed with Pseudo second order r eaction rate model.
[1] APHA, S tandard me tho ds fo r the e xaminatio n o f wate r and waste- wate r, 21st e d., APHA, AWWA, WPCF, Washing to n, D.C, 2005.
[2] R.P.Sing h, N.Gupta, R.S uman and Radha Gupta,‚Re mov al o f He avy me atals fro m Elec tro plating Efflue nts by Carbo nize d ag ro- waste s‛,School of Chemica l Sciences, Ag ra.
[3] ‚Inv e nto ry o f Hazardo us Waste Karnataka state 2007‛_ Ka rna taka
Sta te Pollution Control Board.
[4] H.Zavv ar Mo usav i, S.R.Seye di, ‚Ne ttle ash as a lo w cost adso rbe nt fo r the re mov al o f nic ke l and c admium fro m waste wate r‛, Interna- tiona l Journa l of Environ. Sci. Tech, 8(1), 195-202, 2011.
[5] S.Y.Que k, DAJ Wase and CF Fo rste r-‚The use o f sago waste fo r the so rptio n o f le ad and co ppe r‛, Birmingham University , 24(3), 251-256,
1998.
[6] K.O.Olay inka, B.I.Alo and T.Adu-‚So rptio n o f he avy me tals fro m
Elec tro plating e fflue nts by lo w cost adso rbe nts II: Use o f waste Te a,
Coco nut she ll and Coco nut husk‛, Journal of Applied Science,
7(16),2307-2313, 2007.
[7] Nasim Ahmad Khan, Md. Ghazaly S haaban and Mo hdHasruddin Abu Hassan, ‚Re mov al o f He avy me tal using an ine xpe nsive adso r- be nt‛, University Ma laya 2003.
[8] T.P.Dhung ana and P.N.Yadav, ‚De te rminatio n o f Chro mium in Tan- ne ry Efflue nt and study o f Adso rptio n o f Cr(V I) o n S awdust and Charco al fro m S ug arc ane Bag asses‛, J.Nepa l Cem. Soc., 23, 93-101,
2008/2009.
[9] S riniv asan K. Balasubramaiam N. and Ramakrishna T.V., ‚Chro mium
Re mov al by Rice Husk Carbo n‛, India n Journa l Environmenta l Hea lth,
30(4), 376-387, 1998.
[10] ParindaS uksabye , PaitipThirav e ty an and Wo rananNakb anpo te -
‚Tre atme nt o f chro mium co ntaminate d waste wate r by coconut co ir
pith‛_ King Mo ng ’s Unive rsity o f Tec hno logy, Bang kok.
[11] O.Olay inkake hinde , T.Ade tunde Oluwatoy in, and O.Oyey io la Ade- ro nke , ‚Co mparativ e analy sis o f the e ffic ie nc ies o f two lo w cost ad- so rbe nts in the re mov al o f Cr(V I) and Ni(II) fro m aqueo us so lu- tio n‛, Africa n. Journa l of Environmenta l Science a nd Technology , 3(11),
360-369, 2009.
[12] Khan N.A., Ali S.I and Ay ub S., ‚Effec t o f pH o n the Re mov al o f
Chro mium (Cr) (V I) by S ug arc ane bagg asse ‛, Science a nd Tech., 6, 13-
19, 2001.
[13] N. Kannan and T.Vee maraj, ‚De to xific atio n o f to xic me tal io ns by so rptio n o nto ac tiv ate d c arbo n fro m Heve a Brasilie nsis bark- A co m- parativ e study ‛, Glo bal NEST Jo urnal, 12(2), 197 -205, 2010.
[14] Mo hammad Ajmal, R.A. K. Rao , Jame e l Ahmad and Rais Ahmad,
‚The Use o f Te sta o f g ro undnut S he ll (Arac his hy poge a) fo r the A d-
so rptio n o f Ni(II) fro m the Aqueo us sy ste m‛, Jo urnal o f Env iro n.
Scie nce & Engg , 48(3), 221 -224, 2006
[15] M.To rab-Mo stae di, H.Ghassabzade h, M. Ghannadi -Marag hesh, S.J.Ahmadi and H.Tahe ri, ‚Re mov al o f Cadium and Nic ke l fro m Aqueo us so lutio n using e xpande d pe rlite ‛, Bra zilia n Journa l of Chem i- ca l Engineering, 27(02), 299-308, 2010.
[16] B.Thirunav ukkarasu and K. Palanive lu-‚Bioso rptio n o f Cr(V I) fro m
plati ng e fflue nt using marine alg al mass‛_ Indian Jo urnal o f Bio tec h-
no logy, 6, 359-364, 2007.
[17] Iman. Y. EI-S he rif, A. Ashmawy and S. Badr, ‚Bio so rptio n o f Cad- mium and Nic ke l by Nile Wate r alg ae ‛, Jo urnal o f Applie d Sc ie nce Re se arc h, 4(4), 391 -396, 2008.
—————————— ——————————
IJSER © 201 2