International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1152
ISSN 2229-5518
Chemical and Electrochemical synthesis for
some Metal Complexes of 6-Phenyl-2-thioxo-1,2- dihydropyridine-3-carbonitrile Derivatives
Ahmed A. M. El-Reedy and Ragab R. Amin
Abstract— A new metal Chelates of 6-Phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (HL1) and its derivatives, 6(4-Methylphenyl)-2- thioxo-1,2-dihydropyridine-3-carbonitrile (HL2), 6(4-Chlorophenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (HL3) were prepared and yields complexes of the compositions [M(L)R 2R .(HR 2R O)R2R] and [M(L)R 2R .(acetone)(HR2RO)], where M = Cu(II), Co(II), Ni(II) and L is the ligand. Chemical analysis, infrared as well as thermal analysis are presented to confirm the formulation of the complexes. The spectral data show that the ligands are coordinated to the metal via the thioenol sulfur atom and the nitrogen atom of cyano groups.
Index Terms— 6-Phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (HL1), 6(4-Methylphenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile
(HL2), 6(4-Chlorophenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (HL3), complexes, ligand.
1 INTRODUCTION
—————————— ——————————
Pyridine-2-(1H)-thiones, Mercaptopurine and 2(2H)-Pyridinethione Glycosides have been prepared1-13. Most of these compounds have clinical importance and biological activity. We report and complete the chemical synthesis of some M(II) complexes of a new series of pyridinethione ligands as shown in Fig. 1.

CN
N S R H
Ligand R
HL1: H
HL2: CH
R RHL : Cl
Fig. 1. The Structures of Pyridinethione Derivatives
————————————————
• Ahmed A. M. El-Reedy: Basic and Applied Science Department, Faculty of Oral and Dental Medicine, Nahda University, Beni-Suef, Egypt. E-mail: ahmed.reedy78@gmail.com
• Ragab R. Amin: Basic and Applied Science Department, Faculty of Oral and Dental Medicine, Nahda University, Beni-Suef, Egypt.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1153
ISSN 2229-5518
2 EXPERIMENTAL
2.1 Chemicals and Materials
All the chemicals (Aldrich) were subjected to purification before use. The solvents used were reagent grade. DMF (BDH) (Analar), absolute ethanol and methanol (Fluka) were used as supplied.
2.2 Preparation of the Organic Compound
All the organic compounds were previously prepared1 -13. The structure of the ligand was determined by elemental analysis, IR
1H and 13C-NMR spectroscopies.
2.3 Chemical Preparation of the Complexes
Preparation of the Complexes
The complex [Cu(L1) (H O) ].2H O was prepared by the following procedures. An aqueous solution of hot ethanol of the
R2R
R2R
R2R
R2R
ligand, 6-Phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (HL1) (2.12 g, 0.02 mol) dissolved in absolute ethanol solution of
(60 mL) was added to Copper(II) acetate anhydrous (1.82g, 0.01 mol) and stirred for about 2 hrs. The complex [M(L2) (acetone) ]
R2R
R2R
was prepared by the following procedures. An aqueous solution of acetone solution of the ligand, 6(4-Methylphenyl)-2-thioxo-
1,2-dihydropyridine-3-carbonitrile (HL2), (2.26 g, 0.01 mol) dissolved in absolute ethanol solution of (60 mL) was added to
Copper(II) acetate anhydrous (1.82g, 0.01 mol) and stirred for about 2 hrs. The complex [Cu(L3) (H O) ].2H O was prepared by
R2R
R2R
R2R
R2R
the following procedures. An aqueous solution of hot ethanol of the ligand, 6(4-Chlorophenyl)-2-thioxo-1,2-dihydropyridine-3- carbonitrile (HL3), (2.45 g, 0.01 mol) dissolved in absolute ethanol solution of (60 mL) was added to Copper(II) acetate anhydrous (1.82g, 0.01 mol) and stirred for about 2 hrs., leave the reaction mixtures which produced a colored compound overnight. All the precipitates were filtered, washed with ethanol and dried14. The Cobalt and Nickel complexes were prepared in the same way.
3 ELECTROCHEMICAL SYNTHESIS OF THE COMPLEXES
3.1 Materials
Copper and nickel were used as sheets (2 x 2 cm); cobalt was used in the form of rods (Alfa). Acetone (reagent grade) was dried over anhydrous MgSO4. The ligands were prepared following the literature procedures6.
3.2 Electrochemical Procedure
The electrochemical technique was essentially the same as reported previously9-11. A cell unit consisted of a 100 mL beaker containing anhydrous acetone solution of the thione derivative with a platinum cathode and a sacrificial anode (Co, Ni or Cu) immersed in the liquid phase. A
3.3 Electrochemical Synthesis of Cu(L3) .(acetone)
R2R
R2R
Electrolysis of copper into 60 mL of anhydrous acetone solution of the organic ligand HL3 (0.49 g, 2 mmol), 2.5 mg Et NClO
R4R
R4R
dissolved in two drops of water and 40 mA current led to dissolution of 34 mg of Cu during 30 min. (ERfR = 0.5 mol.F ). Since, most of the products are insoluble in the reaction mixture, the collection procedure involved filtration, after which the solid was
washed with diethyl ether. The resulting powder (0.51 g, 100 %) was collected and analyzed as [Cu(L3 ) .(acetone) ].
4 SPECTRAL, ANALYTICAL AND PHYSICAL MEASUREMENTS
4.1 IR, Raman and 1H-NMR spectra
R 2R
R 2R
Infrared spectra for the samples were recorded by Perkin Elmer FTIR 1605 using KBr pellets. The 1H NMR spectra were recorded on an Varian Mercury VX-300 NMR spectrometer. 1H-NMR spectra were run at 300 MHz and 13C-NMR spectra were run at 75.46 MHz in deuterated dimethylsulphoxide (DMSO-dR6R). Chemical shifts are quoted in δ and were related to that of the solvents.
4.2 Electronic spectra
The electronic spectra of solutions were measured in UV/Vis range (190-1100) nm using UV Spectrometer at Nahda University.
4.3 Magnetic Molar conductance measurements
Magnetic measurements were carried out on a Sherwood Scientific magnetic balance using Gouy method. Molar conductivities of freshly prepared 1.0×10-3 mol L-1 DMSO solutions were measured using Jenway 4010 conductivity meter.
4.4 Microanalytical, Magnetic and Molar measurements
Carbon and hydrogen contents were determined using a Perkin-Elmer CHN 2400 analyser. Magnetic measurements were carried out on a Sherwood Scientific magnetic balance using Gouy method. Molar conductivities of freshly prepared 1.0×10-3 mol/dm-3 solutions of the complexes in DMSO were measured using Jenway 4010 conductivity meter.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1154
ISSN 2229-5518
5 RESULT AND DISCUSSION
The preparative results show that the direct electrochemical oxidation of the metals in the presence of a ligand solution is a one- step process and represents a convenient and simple route to a variety of main group and transition metal complexes. Measurements of the electrochemical efficiency, Ef , defined as moles of metal dissolved per Faraday of electricity, for the M/L system (where M = Co, Ni, Cu and L = ligand used) gave Ef = 0.5 ±0.05 mol F-1. The values listed in Table I show that the reaction of the pyridinethione ligands with copper, cobalt and nickel anodes is compatible with the following equations14-19.
Cathode: 2HL + 2e → 2L- + H2 (g ) (1) Anode: 2L- + M → ML2 + 2e (2)
The pyridinethione derivatives may be represented by the two tautomeric forms i.e., the thione form (I) and thiol form (II),
in Fig. 2.


CN CN
N S SH
(I) Thione form (II) Thiol form
Fig . 2. Tautomeric Forms of Pyridinethione Derivatives.
5.1 Elemental analysis of pyridinethions
The isolated complexes of the pyridinethione derivatives were characterized by elemental analyses, IR, TG and DTG measurements. The results of the elemental analyses are in good agreement with the calculated values and the data are summarised in Table II. The complexes are stable in air and do not melt easily, are insoluble in H2 O, CH3 OH, C2 H5 OH, CHCl3 and soluble in acetone, dimethyl formide (DMF) and dimethyl sulfoxide (DMSO).
6-Phenyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile : Orange crystals, (78%), crystallized from dioxane, m.p. 248◦C, IR (ν cm-1): 3163 (NH), 3058 (aromatic-CH) and 2225 (CN); MS(z/m) = 212 (M+, 100% which corresponds to the molecular weight), 211 (M+-H, 78%); 179 (M+-SH, 18.2%); 1HNMR (DMSO-D6 ) (δppm): 7.228-7.722(m, 5H, Ar H’s), 8.183-9.021 (m, 2H, pyridine H,s) and 14.23 (s, br, 1H, SH); MS (z/m) = 212; Anal. for C12 H8 N2 S (212) Calcd./Found (%): C(67.90/67.91), H(3.80/3.82), N(13.20/13.23), and S (15.11/15.13).
6(4-Methylphenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile : Red crystals, (67%), crystallized from ethanol, m.p. 180◦C, IR (ν cm-1): 3178 (NH), 3065 (aromatic-CH) and 2226 (CN); MS (m/z) = 226 (M+, 100% which corresponds to the molecular weight), 225 (M+-H, 69%); 193 (M+-SH, 17.1%); 1HNMR (DMSO-D6 ) (δppm): 2.123 (s, 3H, CH3 ), 7.205-7.728 (m, 4H, Ar H’s),
8.273-9.032 (m, 2H, pyridine H,s) and 14.14 (s, br., 1H, SH); Anal. for C13 H10 N2 S (226) Calcd./Found (%): C(69.00/69.03), H(4.45/4.43), N(12.38/12.40), and S (14.17/14.20%).
6(4-Chlorophenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile: Orange crystals (70%), crystallized from ethanol, m.p.
170◦C, IR (ν cm-1): 3172 (NH), 3069 (aromatic-CH) and 2221 (CN); MS (m/z) = 246 (M+, 100% which corresponds to the molecular weight), 245 (M+-H, 84%); 213 (M+-SH, 12.5%); 1HNMR (DMSO-D6 ) (δppm): 7.209-7.721(m, 4H, ArH’s), 8.271-
9.028 (m, 2H, pyridine H’s) and 14.17 (s, br., 1H, SH); Anal. for C12 H7 Cl N2 S (246) Calcd./Found (%): C(58.42/58.45), H(2.86/2.89), N(11.35/11.37), Cl(14.37/14.40) and S(13.00/13.03).
5.1 Infrared Spectra
A comparison of the infrared spectra of the pyridinethiones and their metal complexes show the following:
a. The band at 3314.5 and 3200 cm-1 due to ν(NH) in the ligand spectrum is absent in the spectra of the complexes indicating thioenolization.
b. The band at 872.1 cm-1, assigned to ν(C=S), is absent in the spectra of the complexes indicating the rearrangment of C=S
via thioenolization12.
c. The 1578.6 and 1557.8 cm-1 bands [(thioamide III), ν(CN) + ν(CS)] are absent in the complexes.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1155
ISSN 2229-5518
d. The shift to lower frequency of the cyano group by 10-15 cm-1 may suggest the participation of the nitrogen atom in complexation. All of these observations may suggest the participation of the thiol sulphur and the nitrogen of the cyano group in bonding as well as the release of a proton14 on coordination of the pyridinethione ligands with the metal ions in a molar ratio M:L = 1:214. Most of the complexes exhibit ν(OH) and σ(H2 O) bands in the 3400-3450 and 680-690 cm-1 regions which are indicative of coordinated water in these complexes14-20. The non-ligand bands appearing at 440-450 and 410-420 cm-1 in the complex spectra are tentatively assigned to ν(Cu - S)15-18 and ν(Cu ← N)15, respectively.
5.2 Electronic Spectra
The electronic spectrum of [Cu(L1)2 (H2 O)2 ].H 2 O has bands characteristic for an octahedral geometry15-23. The spectrum shows two bands at 17,850 and 19,230 cm-1 assigned to the 4T 1 g → 4A2 g (ν 2 ) and 4T 1 g → 4T 1 g (P) (ν 3 ) transitions, respectively, in an octahedral structure. These bands were used to calculate the third spin-allowed band, 4T 1 g → 4T 1 g17-23. The value of ν 1 was found to be 9,520 cm-1 . The shoulder at 25,000 cm-1 is due to a charge transfer transition, probably, HL6
→ Cu(II)16. The crystal field splitting energies, Δo in kilojoules per mole for Cu(II) metal complexes can be calculated from the following relation: (16)
Δo (kJ/mol) = hc N / λ
where h = 6.626 x 10-34 (J.s), c = 3.00 x 108 (m/s), λ = wavelength (m) and N = 6.023 x 1023 (ions/mol). The calculated values of the crystal field splitting energies, Δo , were tabulated in table.116. The values of the crystal field splitting energies, Δo indicates that the size of the central metal ion affect on the degree of splitting of the d orbitals16.
The electronic spectrum of [Ni(L2)2 .(H2 O)2 ].4H2 O shows a broad band centered at 18,020 cm-1 attributed to the 3A2 g
→ 3T 1 g (F) transtion (ν 2 ). The other shoulder band, (ν 3 ), was observed at 22,988 cm-1 related to the 3A2 g → 3T 1 g (P) transition. The experimental values were used to calculate 3A2 g → 3T 2 g by the d8 equations reported for octahedral structures17-19 and it was found to be 10,073 cm-1. The parameters B, β, 10Dq and the ν2 /ν 1 values were calculated to be 778 cm-1, 0.75, 673, 8 cm-1 and 1.78, respectively. These data confirm the suggested octahedral structure for Ni(L5)2 .(H2 O)2 ].4H2 O.
The electronic spectrum of [Co(L2)2 (H2 O) 2 ].H2 O has bands characteristic for an octahedral geometry17-19. The spectrum shows two bands at 17,850 and 19,230 cm-1 assigned to the 4T 1 g → 4A2 g (ν 2 ) and 4T 1 g → 4T 1 g (P) (ν 3 ) transitions, respectively, in an octahedral structure. These bands were used to calculate the third spin-allowed band, 4T 1 g → 4T 1 g12,17-20. The value of ν 1 was found to be 9,520 cm-1 . The shoulder at 25,000 cm-1 is due to a charge transfer transition, probably, HL6
→ Co(II)16. The other ligand field parameters, B, β, 10Dq and the ν2 /ν 1 values were calculated to be 906 cm-1, 0.88, 9,065 cm-1 and 1.87, respectively, and are in good agreement with those reported for octahedral Co(II) complexes.
The electronic spectrum of [Cu(L3)2 .(H2 O)2 ].4H2 O shows weak shoulders at 18,370 and 13,300 cm-1. The observed bands are due to 2B1 g → 2Eg and 2B1 g → 2A1 g transitions, on the basis of which a distorted octahedral geometry is suggested17-19. The broadening of the bands may be due to the Jahn-Teller effect18-20. However, the 23,364 cm-1 band is due to a d-π* transition20.

Complex Δo , Δo υ1 υ2 υ3 υ2 /υ1 B (cm-1) β
(cm-1) (KJ/mol)

(calculated)
[CoL1 (H O) ].2H O R2R R2R R2R | 11,950 | 130.08 | 10,900 | 23,500 | 29,850 | 2.16 | 1040 | 1.10 |
[Co L2 (H O) ].H O R2R R2R R2R | 11,740 | 127.79 | 14,300 | 23,800 | 32,600 | 1.66 | 900 | 0.86 |
[Co L3 (H O) ].H O R2R R2R R2R | 11,700 | 127.34 | | 25,000 | 30,000 | | | |
[Co L4 (H O) ] 11,695 127.30 - - 30,300 - - -
R2R
R2R
[Co L5 (H O ) ] 11,720 127.64 10,700 22,900 30,500 2.15 1100 1.10
R2R
R2R
[Co L6].H O 11,680 127.19 - - 29,850
[Cu L1 (ac)(H O) ].H O R2R R2R R2R | 00,000 | 127.94 | 14,300 | 23,800 | 32,600 | 1.66 | 900 | 0.86 |
[Cu L2 (ac)(H O) ].H O R2R R2R R2R | 00,000 | 125.58 | 14,300 | 23,800 | 32,600 | 1.66 | 900 | 0.86 |

Table I. The crystal field splitting energies, ΔRoR and some electronic ligand field parameters for Co(II) and Cu(II) complexes of Pyridinethione Derivatives.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1156
ISSN 2229-5518
5.3 Thermal Studies
The thermal behavior of the Cu(II) complexes of some of the pyridinethione derivatives were studied by thermogravimetric
(TG) and differential thermal analysis (DTA). The TG thermaograms of [Cu(L3)2 .(H2 O) 2 ] show a weight loss at 190 °C of
6.0% (calc. 5.4%) corresponding to loss of water from the coordination sphere of the complex. The anhydrous Cu(II) thione complex begins to decompose at 190 °C, a process continuing up to 800 °C through a series of exothermic peaks at 231, 370,
501 and 698 °C, corresponding to decomposition of the organic ligand (calc. 78.8%; found 79.2%)16 - 22. Thermoanalytical methods, such as thermogravimetry, are excellent tools to follow the thermal decomposition of the complexes. The weight percent present at each interval temperature (i.e., every 20 °C) in the TG run was determined and used to calculate the activation energies, ∆E, in each of the weight loss regions. It may be obtained from the following equation22-25
dw/dt = Awn e-∆E/RT
Where A is a constant, E is the activation energy, n is the order of transition, R is the universal gas constant and T is the absolute temperature. According to the above equation, plots of ln(dw/dT) against 1/T for the Cu-complexes of pyridinethione derivatives result in straight lines. The activation energy may be estimated from the slope. The difference in the energy of activation may be due to various factors25, mainly to differences in the electron withdrawing or electron donationg groups in the molecules of the pyridinethione derivatives. In general, the effect of substituents on the hetero ring system and also on the phenyl group will lead to a decrease of cohesive forces and, consequently, will lead to a decrease of the activation energy.
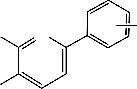
Z

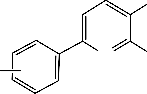
CN S N R
M
N S NC R
Y
M = Co(II), Cu(II) or Ni(II) , R = H, CH3 , Cl
Compound | M | Y | Z |
[M(L1)2 (H 2 O)2 ] [M(L1)2 (H 2 O)2 ].4H2 O [M(L1)2 (acetone)2 ] | Cu Co Ni | H2 O H2 O CH3 COCH3 | H2 O H2 O CH3 COCH3 |
[M(L2)2 (H 2 O)2 ] [M(L2)2 (H 2 O)2 ].4H2 O | Cu Ni | H2 O H2 O | H2 O H2 O |
[M(L3)2 (acetone)2 ] [M(L3)2 (H 2 O)2 ].H2 O | Cu Co | CH3 COCH3 H2 O | CH3 COCH3 H2 O |
[M(L3)2 (acetone)2 ] | Ni | CH3 COCH3 | CH3 COCH3 |
Fig. 3. Suggested Structures for some of the isolated Complexes.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1157
ISSN 2229-5518
REFERENCES
[1] Fawzy A. Attaby, Ahmed H. El-Ghandour, Abdelwahed R. Sayed, Ashraf A. El Bassuony & Ahmed A.M. El-Reedy. Journal of
Sulfur Chem., 33, 197–221, 2012
[2] Elgemeie, G. E. H.; El-Ezbawy, S. E.; Ali, H. A.; Mansour, A. K. Bull. Chem. Soc. Jpn., 67(3), 738-741, 1994.
[3] Elgemeie, G. E. H.; Elzanate, A. M.; Mansour, A. K. J. Chem. Soc., Perkin Trans. 1.,1073-1074, 1992.
[4] Elgemeie, G. E. H.; Attia, A. M. E.; Elzanate, A. M.; Mansour, A. K. Bull. Chem. Soc. Jpn., 67(6), 1-6, 1994.
[5] Elgemeie, G. E. H.; Hussein, B. A. A.,Tetrahedron, 50(1), 199-204, 1994.
[6] Elgemeie, G. E. H.; Regaila, H. A.; Shehata, N. J. Chem. Soc. Perkin Trans. 1. 1267-1270, 1990.
[7] Elgemeie, G. E. H.; Elghandour, A. H.; Ali, H. A.; Hussain, A. A., J. Chem. Res.(S)., 260-261, 1997.
[8] Elgemeie, G. E. H.; Fathy, N. M.; Faddah, L. M.; Ebeid, M. Y., Arch. Pharm. (Weinheim, Germany), 324, 149-152, 1991.
[9] G.H.Elgemeie, A.H.Elghandour, G.W.Abd Elaziz, Synthetic Communications: An International Journal for Rapid Communication of
Synthetic Organic Chemistry, 37, 17, 2827, 2007.
[10] G. H. Elgemeie, A. H. Elghandour, A. M. Elzanate, W. A. Masoud , Phosphorus, Sulfur, and Silicon and the Related Elements, 163, 1,
91, 2000.
[11] G.H.Elgemeie, H.A.Ali, A.H.Elghandour, H. M.Abdel-aziz, Phosphorus, Sulfur, and Silicon and the Related Elements, 170, 1, 171, 2001.
[12] G. E. H. Elgemeie; A. M. E. Attia; S. S. Alkabai, Nucleosides, Nucleotides and Nucleic Acids, 19, 4, 723, 2000.
[13] G. H. Elgemeie, W. A. Zaghary, K. M. Amin; T. M. Nasr, Nucleosides, Nucleotides and Nucleic Acids, 24, 8, 1227, 2005.
[14] Amin, R. R.; El-Gemeie, G. E. H., Synth. React. Inorg. Met.-Org. Chem., 31(3), 431-440, 2001.
[15] Ragab R. Amin, Yamany B. Yamany, Mohamed M. Abo-Aly and Ali M. A. Hassan, Quimica no Brasil, Dec. 21, 2010.
[16] Ragab R. Amin, Journal of Phosphorus, Sulfur, and Silicon and the Related Elements, vol, 185, 537-543, 2010.
[17] Ragab R. Amin, Yamany B. Yamany, Mohamed M. Abo-Aly and Ali M. A. Hassan, Natural Science, vol. 3, No.9, 783-794, 2011.
[18] Ragab R. Amin and Yamany B. Yamany, Journal of Molecular Structure, 1008, 54-62, 2012.
[19] Ragab R. Amin, Yamany B.Yamany, Mohamed Abo-Aly, Ali M. Hassan , Journal of Chemica Acta, 2, 32 – 42, 2013.
[20] Moamen Refat, Fathi Al-Azab, Hussen Mudamma, Ragab R. Amin, Yasmine Jameel, Journal of Molecular Structure 1059, 208–224,
2014.
[21] Lever, A. B. P. Inorganic Electronic Spectroscopy, Elsevier Publishing Company, Amsterdam, 318-361, 1988.
[22] Ragab R. Amin, Taj Al-ansi, Fathi Al-Azab and Ahmed A.M. El-Reedy International Journal of Advanced. Research, 3(3), 562-571,
2015.
[23] El-Asmy, A. A.; Al-Ansi, T. Y.; Amin, R. R.; Mounir, M. M. Polyhedron, 9(17), 2029-2034, 1990.
[24] Nicholls, D. Complexes and First-Row Transition Elements, Reprinted, Great Britain, Macmillan Education LTD, 85-89, 1986.
IJSER © 2015 http://www.ijser.org




