International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 63
ISSN 2229-5518
Characterization of Durable Resistance gene
Yr18/Lr34 against Stripe Rust (Puccinia striiformis
f. sp. tritici) in different Pakistani Wheat Cultivars by Using Molecular (STS) and Morphological (LTN) Markers
Naimat Ullah1*, Tasmia Bashir2, Muhammad Asif1, Hussain Badshah2, Abdul Samad Mumtaz2
94, Pasban-90, Kiran-95, Haider-2000) possessed the desirable marker. Same varieties showed susceptibility at seedling stage and moderate resistance to resistance (MRR) at adult stage under field conditions with either presence or absence of LTN as a morphological marker. The slow-rusting gene, Yr18, can be utilized in combination with other slow-rusting genes to develop high levels of durable adult plant resistance (APR) to stripe rust in wheat.
—————————— ——————————
Wheat (Triticum aestivum L.em.Thell) is chief among the cereals (wheat, maize, rice), being cultivated worldwide due to having adaptability to
altered climatic conditions. Wheat covers an area, globally, of 215 million ha, 44% (62 million ha) of which is grown in Asian countries like China, India and Pakistan (1). The current wheat production in South Asia is around 95 million tones and demand for 2020 is estimated to be around 137 million tones. Among the most important diseases in wheat that significantly reduce wheat production are those caused by the rusts (leaf, stem, and stripe). Stripe rust caused by Puccinia striiformis West, is most likely the most widely distributed, and infect both
• * 1Naimat Ullah (Corresponding author) is currently pursuing Doctoral degree program in Biological Sciences in Sabanci University, Turkey, PH-+90-553-3641997. E-mail: naimat@sabanciuniv.edu
• 1Sabanci University, Faculty of Engineering and Natural
Sciences, 34956, Istanbul, Turkey.
• 2Quaid-I-Azam University, Department of Plant Sciences,
spring and winter wheat cultivars across diverse cultivated regions in the world (2–5). Various methods have been used to control the stripe (yellow) rust of wheat worldwide which are; plant resistance, use of fungicides and cultural control.
The most efficient, cost-effective and environment-friendly
approach to prevent the losses caused by rust epidemics is the development of genetic resistance to biotic stress. The use of cultivars with single-gene resistance (race-specific resistance) permits the selection of mutations at a single locus to render the resistance effective in a relatively short time. However, due to loss of variation and selection pressure, and evolution, new virulent races of the fungus appear which increase the need to develop durable resistance. Hence, the use of combinations of resistance genes has been suggested as the best method for genetic control of stripe and other rusts. This can be achieved by pyramiding effective resistance genes, but expression of individual resistance genes is difficult to monitor in the field. Conversely, a group of race-nonspecific resistance
45320, Islamabad, Pakistan.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 64
ISSN 2229-5518
mechanisms has been described in wheat which is mainly polygenic. This has often been described as slow rusting (6), adult plant resistance and partial resistance (5,7) and is known to be long-lasting and more durable (8,9).
A more durable resistance to stripe rusts involves slow rusting (10,11) which is defined as a form of partial resistance in which host genotypes delay rust development (12) through different mechanisms (13). In many cereal-rust patho-systems, the quantitative aspects of cultivar resistance have been described and estimated by means of disease severity at a certain crop development stage, the area under disease progress curve (AUDPC) and Coefficient of Infection (CI) values for adult plant resistance (14–16). Two loci, Lr34/Yr18 complex on chromosome 7DS (17) and the Lr46/Yr29 complex on 1BL (18,19), express resistance to both leaf (brown) (P. triticina) and yellow rusts. The Lr34/Yr18 locus, in particular, is of immense importance since it has contributed to durable resistance against the two rust pathogens. Both types of resistance have always been inherited jointly and reported to be either pleiotropic or completely linked (2,18,19). This gene pair is reported to co-segregate with other traits such as leaf tip necrosis, powdery mildew (designated as Pm38), and tolerance to Barley yellow dwarf virus (Bdv1) (20,21).This multi-pathogen protection provided by Yr18/Lr34 locus has made it one of the most valuable genetic regions for disease resistance wheat breeding.
However, information on slow rusting resistance conferred by Yr18/Lr34 complex in Pakistani wheat was not available. Therefore, this study is conducted to characterize 30
Pakistani wheat cultivars for slow rusting resistance at both
phenotypic and genotypic levels and to test the efficiency of
different epidemiological parameters in selecting slow rusting genotypes.
Cereal Crops Research Institute (CCRI) Pir Sabaq, (Noshehra KPK, Pakistan) was selected as an experimental site because yellow rust come to occurs more severe over there every year (22) and over-summering is frequent in the region. Therefore, this location is known as hotspot for the yellow rust (23). Being situated at the gateway of the new rust races entering from neighboring, this area has got geographic distinctiveness (Coordinates: 34°0'55N
71°58'29E) in Pakistan. A set of thirty selected Pakistani
wheat varieties were grown at CCRI along with a
susceptible control Morocco as shown in Table. 1. Each variety was planted in 2m long row with 30 cm distance
between the rows. Mixture of Morocco was sown around the trial as a spreader row to make possible development of rust epidemic.
Data for disease severity and infection types were assessed in the field at least thrice during adult plant stages: firstly, when the first spikelet was visible, then at the peak period of disease development when the susceptible control Morocco reached a disease severity of 100S and lastly at maturity. The severity was recorded as percent of the rust infection on the plants according to the modified Cobb scale (24) incorporating both percent leaf area affected and the host response (Table 2).
DNA was extracted from 10 days-old seedlings of 19 cultivars (only MRMS cultivars) by the CTAB method (25). Fresh leaves were cut from the plants and placed in 1.5 ml eppendorf tubes. The tubes were subsequently dropped in liquid Nitrogen for rapidly freezing the leaf material. The plant material was then crushed with a micro pestle while inside the tube by adding 1 ml of preheated (65 ºC) 2X CTAB solution. The homogenized leaf tissues were transferred to two 1.5 ml eppendorf tubes and were incubated in a water bath at 65 ºC for 30 min. 0.5 ml of chloroform and isoamyl-alcohol (24:1) were added and were inverted vertically 5-10 times, followed by centrifugation at 10,000 rpm for 10 minutes. After centrifugation, supernatant was transferred to fresh tubes and 0.6 volume of 3M sodium acetate was added. Then
500µl cold isopropanol was added and mixed properly by inverting the tubes a few minutes. The DNA was pelleted and washed with 70% cold ethanol. The pellet was air dried and re-suspended in 40µl 0.1X TE buffer. (Purified DNA samples were stored at -20°C for further use).
Purity of the dissolved DNA in the samples was analyzed by the checking the absorbance ratios at 260/280 nm on spectrophotometer while concentration was calculated assuming that 1 O.D. (optical density) at 260 nm corresponds to 50ng/µl of DNA. Screening of twenty wheat varieties was carried out using STS marker (csLV34) linked to stripe rust resistance gene Yr18 (Gene link, NY USA (Table 3).The 10µl reaction mixture consisted of 60-70ng of template DNA, 1.0 µl Mg-free 10 X PCR Buffer (Fermentas),
0.6µl (5unit/µl) of Taq DNA polymerase (Fermentas), 25
mM of MgCl2 , 2.5 mM dNTPs (Sigma Chemical Co., St.
Louis, MO) and 30ng of a single primer synthesized by
Gene link (NY, USA). After 5 min of denaturation at 94°C,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 65
ISSN 2229-5518
amplifications were programmed for 40 consecutive cycles each consisting of 1 min at 94°C, 1 min at 55°C annealing, 2 min at 72°C followed by a 7 min extension step at 72°C. A total of nineteen varieties were studied for the identification of stripe rust resistance genes using STS markers reported to be linked with stripe rust resistance genes. Morocco susceptible was used as negative control while Chinese spring with known gene, Yr18 for stripe rust resistance were used as a positive check. The sequence of STS marker Yr18 is shown in Table 3.
The disease severity data were used to calculate the area under the disease progress curve (AUDPC) using a computer program developed at CIMMYT. The relative percentage of area under the disease progress curve for each entry was calculated by setting AUDPC of Morocco as
100% (5).
Thirty selected Pakistani wheat genotypes were evaluated for stripe rust resistance at the adult plant stage after natural infection in Pir Sabaq (Nowshera KPK, Pakistan). Disease observations were recorded as reaction types of stripe rust and its severity on affected leaves of the diseased plants.
Based on reaction type (26) and severity measured in percentages (27); of the thirty wheat varieties, 3 (10%) of the genotypes (ZA-77, Soghat-90, Rawal-87) were resistant (with infection type (IT) R, severity 1-10%), 19 (63.34%) cultivars (C-518, mexipak, chenab-70, kohinoor-83, faisalabad-83, rohtas-90, bakhtawar-93, zardana-93, kaghan-93, shahkar-95, moomal-2002, punjab-96, bahawalpur-95, haider-2000, pasban-90, sarsabz, anmol, wattan-94 and kiran-95 and Pasban-90) were intermediates or moderately resistant to moderately susceptible (with IT MRMS, severity 11-20%), 3(10%) genotypes (Suleman-96, Kohsar-95 and Khybar-87) were moderately susceptible to susceptible (with IT MS-S, severity 21-30%) and 4 (13.34%) of genotypes (INQ-91, Kohistan-97, Punjnad-88 and Morocco) were susceptible (with IT S, severity 31-100%) (26) as shown in Figure 1 and Figure 2.
Based on derived Coefficient of Infection values, Cultivars with CI values of 0-10, 11-20 and above 20 were regarded as possessing high, moderate and low levels of adult plant resistance, respectively. Results revealed that 3 (4%) cultivars (ZA-77, SOGHAT-90 and RAWAL-87) showed high level of adult plant resistance with coefficient of infection values between 0-10, 19 (70%) cultivars (C-518, Mexipak, Chenab-70, Kohinoor-83, Faisalabad-83, Inq-91, Rohtas-90, Soghat-90, Bakhtawar-93, Zardana-93, Kaghan-
93, Shahkar-95, Suleman-96, Kohistan-97, Moomal-2002, Punjab-96, Bahawalpur-95, Kohsar-95, Haider-2000) displayed moderate level of adult plant resistance and coefficient of infection ranged between 11-20. Low level of adult plant resistance shown by remaining 8 (30%) cultivars (Suleman-96, Kohsar-95, Khybar-87, Pirsabak-2004, Kiran-
95, Khybar-87, Sarsabz and Punjnad-88) with CI values
above 20 as shown in Figure 3.
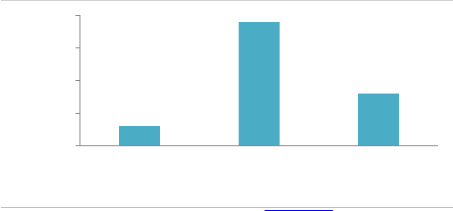
Based on rAUDPC values (26), Cultivars were categorized into three distinct groups. The first group included genotypes exhibiting rAUDPC values up to 10% of check were considered resistant. While genotypes showing rAUDPC values 11 to 20% of check were placed in second group of moderately resistant to moderately susceptible Cultivars. Third group comprised of genotypes with rAUDPC values above 20% of the check were rated as susceptible. Results revealed that, 3(%) Cultivars (ZA-77, Soghat-90 and Rawal-87) were resistant (Raudpc 1-10%). While 12(%) cultivars (C-518, Mexipak, Chenab-70, Faisalabad-83, INQ-91, Rohtas-90, Soghat-90, Bakhtawar-93, Zardana-93, Shahkar-95, Suliman-96, Kohistan-97, Punjab-
96, Bahawalpur-95, Kohsar-95, Moomal-2002, Kiran-95,
Sarsabz, Rawal-87 and wattan-94) were moderately resistant to moderately susceptible ( rAUDPC 11-20%) . Remaining three Cultivars (Punjab-96, Bahawalpur-95, Kohsar-95) were susceptible (rAUDPC>20%) as shown in Figure.4.
Leaf tip necrosis, a morphological marker (28) was recorded in wheat varieties after heading stage under field conditions. In present study, of 30 selected Pakistani wheat varieties, 18 Cultivars (C-518, Mexipak, Chenab-70, ZA-77, Kohinoor-83, Soghat-90, Kohsar-95, Haider-2000, Pirsabak-
2004, Kiran-95, Khybar-87, Sarsabz, Punjnad-88, Rawal-87, Anmol, Wattan-94 Pasban-90 and Morocco) lacked LTN phenotype. While remaining 12 genotypes (Bahawalpur-95,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 66
ISSN 2229-5518
Bakhtawar-93, Zardana-93, Suleman-96, Punjab-96, Faisalabad-83, Inq-91 and Rohtas-90) expressed LTN as shown in Figure 5.
Out of 30 wheat varieties evaluated against stripe rust under field conditions, a sub set of 20 Cultivars with reaction types MRMS, were selected for molecular Characterization. STS marker (csLV34F/csLV34R) was used for a specific gene Yr18 in Pakistani selected wheat germplasms. The STS marker showed polymorphism for Yr18 in wheat genotypes. Results revealed that STS marker csLV34 amplified two alleles, a band of 150 bp size that has been reported to be tightly linked with resistant gene Yr-18 and another fragment of 230 bp size, not associated with resistance shown in Table 5. Cultivars; C-518, Mexipak, Kohinoor-83, Zardana-93, Shahkar-95, Moomal-2002, Haider-2000, Kiran-95, Wattan-94 and Pasban-90 showed amplified band of 150bp and the remaining 10 cultivars such as Chenab-70, Rohtas-90, Bakhtawar-93, Kaghan-93, Punjab-96, Bahawalpur-95, Sarsabz and Anmol amplified a fragment of 230 bp shown in Figure 6.
Seedling and field testing revealed that majority of (total
22) cultivars (C-518, Mexipak, Chenab-70, Faisalabad-83,
INQ-91, Rohtas-90, Soghat-90, Bakhtawar-93, Zardana-93,
Shahkar-95, Suliman-96, Kohistan-97, Punjab-96, Bahawalpur-95, Kohsar-95, Moomal-2002, Kiran-95, Sarsabz, Rawal-87 and Wattan-94) had higher reaction (susceptibility) at seedling stage and were classified as moderately resistant to moderately susceptible at adult plant stage with low rAUDPC (15.2-27.8%), CI reaction type and severity (11–20%). Cultivars carrying slow rusting display high infection type in the seedling and lower rAUDPC at adult stage may have race-nonspecific resistance. Similarly, cultivars with CI values of 0-20, 21-40 and 41-60 at adult stage are regarded as possessing high, moderate and low levels of adult plant resistance, respectively. Based on the seedling and field data, 22 cultivars may have race nonspecific resistance for stripe rust. This type of resistance remains effective for longer time, even if pathogen changes its genotype, because durable resistance, such as slow rusting and High- Temperature Adult Plant resistance (HTAP), is controlled by more than one genes (at least 2-3).
Leaf Tip Necrosis (LTN), a morphological trait, shows complete linkage or pleiotropism in some cultivars with Yr18/Lr34 genes and could be used as a morphological
marker to identify wheat lines carrying these genes (Singh
1992). In the present study its expression was observed
positive for eight varieties (Faisalabad-83, INQ-91, Rohtas-
90, Bahawalpur-95, Zardana-93, Suleman-96, Punjab-96,
and Bakhtawar-93). Varieties with LTN also have been rated as moderately resistant based on phenotypic parameters of rAUDPC, CI and severity of stripe rust at adult stage. However, this parameter carries a few drawbacks. Its expression can be masked by genetic background and variable influences of environments. Shah et al. (2010) evaluated a set of Pakistani wheat cultivars at seedling and adult plant stage for resistance to stripe rust with selected Pak isolates of Puccinia striiformis f. sp. triticiand reported similar results. In our study some varieties have shown leaf tip necrosis but others not which shown durability or slow rusting under molecular marker csLV34, So LTN is not more perfect and trustworthy morphological marker as it can be easily encountered under different environmental stresses. For further validataion, molecular markerfor Yr18 has been used to determine the presence or absence of the gene in these cultivars. STS marker showed ponlymorphism for Yr18 in wheat genotypes. Of thirty, 11 genotypes (C-518, Mexipak, Kohinoor-83, Faisalabad-83, Zardana-93, Shahkar-95, Moomal-2002, Haider-2000, Pasban-90, Wattan-94 and Kiran-95) showed amplification of band of 150 bp reported to be linked with Yr18 (Lagudah et al., 2006). This indicates that these genotypes have gene Yr18. Based on STS marker data, Genetic relationship among 19 wheat genotypes was determined. UPGMA-based dendrogram grouped 19 genotypes into two clusters. 11 genotypes (C-518, Mexipak, Kohinoor-83, Faisalabad-83, Zardana-93, Shahkar-95, Moomal-2002, Haider-2000, Pasban-90, Wattan-94 and Kiran-95) formed a distinct and largest group with closely related genotypes showing amplification of 150 bp (linked to gene Yr18) in first cluster (A). Second cluster (B), with 8 genotypes with 230bp fragment formed the small group. Both these clusters were grouped together at similarity level of 80%.Tabassum et al.,(2010) carried out molecular characterization and reported similar clustering pattern of wheat genotypes based on STS marker data. Our results found similar to both Tabassum et al., (2010) and shah et al., (2010) when compared on the bases of Molecular marker, csLV34, and field data(29).
A special note of thanks to Dr. Abdul Samad Mumtaz, Assisstant Professor Department of Plant Sciences, Quaid-I- Azam University, 45320, Islamabad, Pakistan
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 67
ISSN 2229-5518
Yr: Yellow rust
Lr: Leaf rust
STS: Sequence Tag Specific
LTN: Leaf Tip Necrosis
MAS: Marker Assisted Selection
APR: Adult Plant Resistance
AUDPC: Area under Disease Progress Curve
CCRI: Cereal Crops Research Institute
1. Ali S, Shah SJA, Raman IHKH, Maqbool K, Ullah
W. Southern Cross Journals © 2009 Partial
resistance to yellow rust in introduced winter wheat germplasm at the north of Pakistan. 2009;3(1):37–43.
2. Bahri B, Shah SJ a., Hussain S, Leconte M, Enjalbert J, de Vallavieille-Pope C. Genetic diversity of the wheat yellow rust population in Pakistan and its relationship with host resistance. Plant Pathology.
2011 Aug 27;60(4):649–60.
3. Bonman JM, Bockelman HE, Jin Y, Hijmans RJ, Gironella AIN. Geographic Distribution of Stem Rust Resistance in Wheat Landraces. Crop Science.
2007;47(5):1955.
4. Lagudah ES, McFadden H, Singh RP, Huerta- Espino J, Bariana HS, Spielmeyer W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 2006 Dec;114(1):21–30.
5. Ma Q, Shang HS. ultrastructure of strıpe rust ( puccınıa strııformıs f . sp . trıtıcı ) ınteractıng wıth slow-rustıng , hıghly resıstant , and susceptıble wheat cultıvars. 2009;91:597–606.
6. Ramburan VP, Pretorius Z a, Louw JH, Boyd L a, Smith PH, Boshoff WHP, et al. A genetic analysis of adult plant resistance to stripe rust in the wheat cultivar Kariega. TAG Theoretical and applied genetics Theoretische und angewandte Genetik.
2004 May;108(7):1426–33.
7. Singh RP, Huerta-espino J, William HM. Genetics and Breeding for Durable Resistance to Leaf and
Stripe Rusts in Wheat. 2005;29:121–7.
8. Singh RP, Bhavani S, Singh D, Singh PK. Race non- specific resistance to rusts in CIMMYT spring wheats : Breeding advances. 2010;
9. Mallard S, Gaudet D, Aldeia a, Abelard C, Besnard a L, Sourdille P, et al. Genetic analysis of durable resistance to yellow rust in bread wheat. TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 2005 May;110(8):1401–9.
10. Street ZS, Zhou XC, Winter G, Tan DJ, Niu YC, Agri- CA, et al. Seedling and Slow Rusting Resistance to Stripe Rust in Chinese Common Wheats. 90(10).
11. Venkata BP, Bansal UK, Singh RP, Park RF, Bariana HS. Genetic Analyses of Durable Adult Plant Resistance to Stripe Rust and Leaf Rust in CIMMYT Wheat Genotype 11IBWSN50. 2008;
12. Singh RP, Hodson DP, Huerta-espino J, Jin Y, Njau P, Wanyera R, et al. Will Stem Rust Destroy the World ’ s Wheat Crop ? 2008;98(08).
13. Powell N, Berry S, Boyd L, Ranger M. The genetic characterisation of adult plant resistance to yellow rust in the winter wheat cultivar Claire. 1993;2–4.
14. Martinez A, Youmans J, Buck J. Stripe Rust ( Yellow
Rust ) of Wheat.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 68
ISSN 2229-5518
15. Ayliffe M, Singh R, Lagudah E. Durable resistance to wheat stem rust needed. Current opinion in plant biology. 2008 Apr;11(2):187–92.
16. Johnson J, Buck J, Marshall D, Miranda L, Martinez A. Stripe rust resistance in soft red winter wheat cultivars and lines. :2–3.
17. Martínez F, Niks RE, Singh RP, Rubiales D.
Characterization of Lr46, a Gene Conferring Partial
Resistance to Wheat Leaf Rust. Hereditas. 2004 Apr
22;135(2-3):111–4.
18. Suenaga K, Singh RP, Huerta-Espino J, William HM. Microsatellite markers for genes lr34/yr18 and other quantitative trait Loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology. The American Phytopathological Society; 2003 Jul
22;93(7):881–90.
19. William M, Singh RP, Huerta-Espino J, Islas SO, Hoisington D. Molecular marker mapping of leaf rust resistance gene lr46 and its association with stripe rust resistance gene yr29 in wheat. Phytopathology. The American Phytopathological Society; 2003 Mar 22;93(2):153–9.
20. Suenaga K, Singh RP, Huerta-Espino J, William HM. Microsatellite markers for genes lr34/yr18 and other quantitative trait Loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology.
2003 Jul;93(7):881–90.
21. Wellings C. Global status of stripe rust.
2010;(2002):1–15.
22. Chatrath R, Mishra B, Ortiz Ferrara G, Singh SK, Joshi AK. Challenges to wheat production in South Asia. Euphytica. 2007 Aug 10;157(3):447–56.
23. Bux H, Ashraf M, Hussain F, Rattu A-U-R, Fayyaz M. Characterization of wheat germplasm for stripe rust (’Puccini striiformis' f. sp. “tritici”) resistance. Southern Cross Publishers; 2012 Feb 1;6(1):116.
24. Peterson RF, Campbell AB, Hannah AE. A DIAGRAMMATIC SCALE FOR ESTIMATING RUST INTENSITY ON LEAVES AND STEMS OF CEREALS. Canadian Journal of Research. NRC Research Press Ottawa, Canada; 1948 Oct
13;26c(5):496–500.
25. Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter.
1997 Mar;15(1):8–15.
26. Lal Ahamed M, Singh SS, Sharma JB, Ram RB.
Evaluation of inheritance to leaf rust in wheat using
area under disease progress curve. Hereditas. 2004
Jan;141(3):323–7.
27. Genga A, Mattana M, Coraggio I. Abiotic Stress in Plants - Mechanisms and Adaptations. Shanker A, editor. InTech; 2011.
28. Singh RP. Association between Gene Lr34 for Leaf Rust Resistance and Leaf Tip Necrosis in Wheat. Crop Science. Crop Science Society of America;
1992;32(4):874.
29. Chen X, Moore M, Milus EA, Long DL, Line RF, Marshall D, et al. Wheat Stripe Rust Epidemics and Races of Puccinia striiformis f. sp. tritici in the United States in 2000. Plant Disease. The American Phytopathological Society; 2002 Jan 23;86(1):39–46.
S.No. | Varieties | Pedigrees |
1 | C-518 | T9 x 8A |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 69
ISSN 2229-5518
2 | MEXIPAK | PJ62 ‘S’-GB-55 |
3 | CHENAB-70 | C271-WT(E) x SON64 |
4 | ZA-77 | NORTENO 67 X SIETE CROSS 11 30367-IM-IY-3M-0Y |
5 | KOHINOOR-83 | (OREF1.158-FDL x MEXIFEN ‘S’-TIBA632) COC |
6 | FAISALABAD-83 | FURY x KAL-BB |
7 | INQ-91 | WL711/CROW ‘S’ |
8 | ROHTAS-90 | 1N1A66 ⁄ A ⁄ DISTT ⁄ ⁄ 1N166 ⁄ 3 ⁄ GEN81 |
9 | SOGHAT-90 | PAVON ⁄ SODIUM 223DE |
10 | BAKHTAWAR-93 | KAUZ’S’ |
11 | ZARDANA-93 | CNO S ⁄ 8156 TOB 66 CNO6-PVN |
12 | KAGHAN-93 | TEETER (SIB) ⁄ (SIB) JUNCO |
13 | SHAHKAR-95 | WL711//F3.71/TRM |
14 | SULEMAN-96 | F6.74 ⁄ BUN ⁄ ⁄ SIS ⁄ 3 ⁄ VEE#7 |
15 | KOHISTAN-97 | V-1562//CHRC ‘S’/HORK / 3 / KUFRA-1 /4/CARP ‘S’/BJY |
16 | MOOMAL-2002 | VEE/TRAP#1//SOGHAT-90 |
17 | PUNJAB-96 | INIA/SON64-P.4160(E)XSON64 |
18 | BAHAWALPUR-95 | CNO ‘S’-LR4 X SON 64/SON (AMBER) |
19 | KOHSAR-95 | PSN ‘S’ /BOW ‘S’ |
20 | HAIDER-2000 | CHIL/WUH3 |
21 | PIRSABAK-2004 | KAUZ/STAR |
22 | KIRAN-95 | WL711 NaN3 (MUTANT 7/81) X CROW ‘S’ |
23 | KHYBAR-87 | LIRA’S’ |
24 | SARSABZ | PI-FROND/PI-MAZOE |
25 | PUNJNAD-88 | K.4500*2/BJY |
26 | RAWAL-87 | MAYA-MONCHO ‘S’/KVZ-TRM |
27 | ANMOL | LIRA ‘S’ |
28 | WATTAN-94 | * |
29 | PASBAN-90 | INIA66/A.DISTT//INIA66/3/GEN81 |
30 | MOROCCO | * |
* Pedigree not known.
Reaction/response | Observation |
No disease | O |
Resistant | R |
Moderately Resistant- Resistant | MRR |
Moderately resistant | MR |
Moderately resistant-Moderately Susceptible | MRMS |
Moderately Susceptible | MS |
Moderately Susceptible-Susceptible | MSS |
Susceptible | S |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 70
ISSN 2229-5518
Name of STS marker | Sequance (5'-3') | Gene | Size (bp) | Reference |
csLV34F | GTTGGTTAAGACTGGTGATGG | Yr18 | 150 | Lagudah et al., 2006 |
csLV34R | TGCTTGCTATTGCTGAATAGT | Yr18 | 150 | Lagudah et al., 2006 |
S.No | Varieties | APR | Mean coefficient of infection | Mean of AUDPC | Mean of rAUDPC | |
1 | C-518 | MRMS | 12.5fgh | 36.25efg | 25.84efg | |
2 | MEXIPAK | MRMS | 16.67fgh | 41.09ef | 29.23def | |
3 | CHENAB-70 | MRMS | 17.5fg | 41.73ef | 29.78def | |
4 | ZA-77 | R | 1.83gh | 13.78g | 9.81fg | |
5 | KOHINOOR-83 | MRMS | 20f | 44.63ef | 31.84de | |
6 | FAISALABAD-83 | MRMS | 10.83fgh | 33.81fg | 24.11efg | |
7 | INQ-91 | S | 93.33c | 89.05c | 63.50c | |
8 | ROHTAS-90 | MRMS | 15.83fgh | 39.31ef | 28.05def | |
9 | SOGHAT-90 | R | 1.33gh | 15.39g | 10.93fg | |
10 | BAKHTAWAR-93 | MRMS | 18.33f | 42.86ef | 30.53de | |
11 | ZARDANA-93 | MRMS | 12.5fgh | 36.25fg | 25.84efg | |
12 | KAGHAN-93 | MRMS | 16.67fgh | 41.32ef | 29.48def | |
13 | SHAHKAR-95 | MRMS | 17.5fg | 41.73ef | 29.79def | |
14 | SULEMAN-96 | MS | 41.67e | 61.29de | 43.71d | |
15 | KOHISTAN-97 | S | 153.33b | 110.45b | 78.78b | |
16 | MOOMAL-2002 | MRMS | 15fgh | 39.68ef | 28.25def | |
17 | PUNJAB-96 | MRMS | 15.5fgh | 39.91ef | 28.48def | |
18 | BAHAWALPUR-95 | MRMS | 12.5fgh | 36.25fg | 25.84efg | |
19 | KOHSAR-95 | MS | 58.33d | 70.63d | 50.38d | |
20 | Haider-2000 | MRMS | 20f | 44.63ef | 31.83de | |
21 | PIRSABAK-2004 | 0 | 0h | 9.89 | 7.05g | |
22 | KIRAN-95 | MRMS | 18.33f | 42.22ef | 30.10de | |
23 | KHYBAR-87 | MS | 46.67e | 63.37de | 45.18d | |
24 | SARSABZ | MRMS | 10.83fgh | 33.83fg | 24.10efg | |
25 | PUNJNAD-88 | S | 93.33c | 89.45c | 63.50c | |
26 | RAWAL-87 | R | 1.833gh | 16.82g | 11.66fg | |
27 | ANMOL | MRMS | 18.33f | 42.22ef | 30.10de | |
28 | WATTAN-94 | MRMS | 58.33d | 70.63d | 50.38d | |
29 | PASBAN-90 | MRMS | 13.33fgh | 35.98fg | 25.65efg | |
30 | MOROCCO | S | 233.33a | 140.36a | 100.03a |
Pakistani wheat genotypes to yellow rust. (Mean comparison based on Duncan multiple testing*) *Means followed by the same letters in each column are not statistically significant at p<0.05.
S.No | Varieties | Yr-18 (150 bp) | LTN |
1 | C-518 | Present | Absent |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 71
ISSN 2229-5518
2 | MEXIPAK | Present | Absent |
3 | CHENAB-70 | Absent | Absent |
4 | ZA-77 | NC | Absent |
5 | KOHINOOR-83 | Present | Absent |
6 | FAISALABAD-83 | Present | Present |
7 | INQ-91 | NC | Present |
8 | ROHTAS-90 | Absent | Present |
9 | SOGHAT-90 | NC | Absent |
10 | BAKHTAWAR-93 | Absent | Present |
11 | ZARDANA-93 | Present | Present |
12 | KAGHAN-93 | Absent | Absent |
13 | SHAHKAR-95 | Present | Absent |
14 | SULEMAN-96 | NC | Present |
15 | KOHISTAN-97 | NC | Absent |
16 | MOOMAL-2002 | Present | Absent |
17 | PUNJAB-96 | Absent | Present |
18 | BAHAWALPUR-95 | Absent | Present |
19 | KOHSAR-95 | NC | Absent |
20 | Haider-2000 | Present | Absent |
21 | PIRSABAK-2004 | NC | Absent |
22 | KIRAN-95 | Present | Absent |
23 | KHYBAR-87 | NC | Absent |
24 | SASABZ | Absent | Absent |
25 | PUNJNAD-88 | NC | Absent |
26 | RAWAL-87 | NC | Absent |
27 | ANMOL | Absent | Absent |
28 | WATTAN-94 | Present | Absent |
29 | PASBAN-90 | Present | Absent |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 72
![]()
ISSN 2229-5518
30 MOROCCO NC Absent
NC= Not Characterized
80.00%
63.34%
60.00%
40.00%
20.00%
0.00%
10% 13% 13.34% MRMS MS-S S R
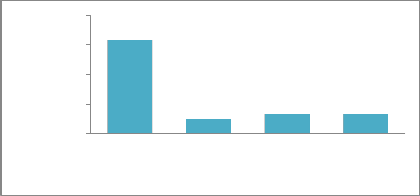
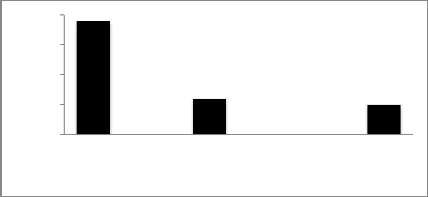
20 19
15
10
6 5
5
0
0-30 31-60 70-100
20
15
10
5
0
High APR Moderate APR Low APR
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 73
ISSN 2229-5518
70%
60%
50%
40%
30%
20%
10%
0%
10%
60%
26.67%
0-10 11−20 21─100
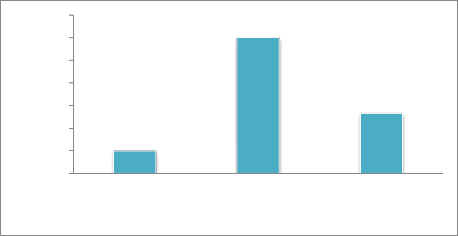
20 18
15
12
10
5
0
Present Absent
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 74
ISSN 2229-5518
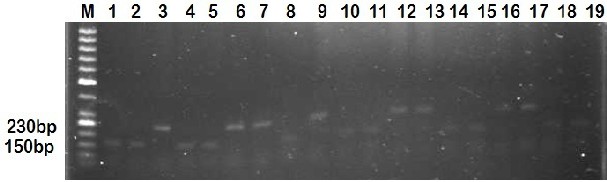
is indicated by the arrow. Products were separated on a 2% agarose gel. 1: Yr-18 source stock (Chinese spring), 2: Mexipak 3:
Chenab-70, 4: Kohinoor-83, 5: Faisalabad-83, 6: Rohtas-90, 7: Bakhtawar-93, 8: Zardana-93, 9: Kaghan-93, 10: Shahkar-95, 11: Moomal-2002, 12: Punjab-96, 13: Bahawalpur-95, 14: Haider-2000, 15: Kiran-95, 16:Sarsabz, 17: Anmol, 18: Wattan-94, 19: Pasban-
90.
IJSER © 2015 http://www.ijser.org