International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 112
ISSN 2229-5518
Characterization and Phytochemical Screening of n- Hexane Oil Extract from Cissus aralioides Seeds
Akinsanmi A. Oduje, Anthony Awode, Alexander Edah, and, Itse Sagay
ABSTRACT- The aim of this research work is to investigate the physico-chemical properties and phytochemical screening of cissus aralioides oil using solvent extraction method, with n-hexane as the extracting solvent. The Phytochemical analysis was determined using GC-MS, which reveal the presences of Diethyl Phthalate; 6-Octadecenoic acid and Octadecanoic acid, 2-(2-hydroxyethoxy) ethyl ester. The phytochemical screening shows a positive result for alkaloids and steroids. The AOAC method of Analysis was employed in the determination of the physico-chemical properties of the oil. The chemical properties of the oil determined include saponification value, free fatty acid, iodine value, peroxide value and acid value. The physical properties of the oil determined are specific gravity, refractive index, cold test. The values obtained are; Saponification value (130.58 ± 0.30mgKOH/g]]free fatty acid (2.13 ± 0.025mgKOH/g), iodine value (80.74 ± 1.53gI/100g), peroxide value (34.67 ± 0.10mEq/kg), acid value (4.24mgKOH/g), Relative density (0.9220),Refractive index at 25 ◦C (1.4758 ± 0.0002) , and cold test (Low Turbidity). The moisture content of the seeds was 25.1 ± 0.18 and the oil yield was 23 ± 0.55. From the results obtained it can be seen that cissus aralioides oil, has a great potential in cosmetic, soap and pharmaceutical industries.
Index terms: Cissus aralioides, N-hexane, phytochemical screening, phytochemicals, Physiochemical properties, oil properties, medicinal plant
—————————— ——————————
Plants are endowed with various phytochemical molecules such as vitamins, terpenoid, phenolic acids, lignins, stilbenes, tannins, flavonoids, quinones, coumarins, alkaloids, amines, betalinins, and other metabolites, which are rich in antioxidant activities [1]. However recent Epidemiological and clinical studies shows that plant oil reduces the risk of Alzheimer’s disease stroke, inflammation and certain types of cancer [2] [3]. Oils that are extracted from plant source are referred to as plant oils and are liquid at room temperature [4]. This plant oils were long perceived to be unhealthy due to their high fat content and caloric value [5]. The screening of plant oil has shown it to contain bioactive lipid component such as monosaturated and polysaturated fatty acid and they are used as active ingredient or excipient in cosmetics, pharmaceuticals, and soap industries [6]. Caprylic acid, oleic acid and phthalic acid are found in plants oil, they are used as disinfectants [7], anti-microbial [8] and anti-bacterial [9].
Phytochemicals (from the Greek word phyto, meaning plant) are biologically active, naturally occurring chemical compounds found in plants, which provide health benefits for humans further than those attributed to macronutrients and micronutrients [10]. It is well-known fact that plants produce these chemicals to protect themselves, but recent research demonstrate that many phytochemicals can also protect human against diseases [11]. Phytochemical analysis also shows the presence of alkaloids, tannins, phenolics, saponins, glycoside, steroids and terpenoids [12].
Cissus aralioides is a vine plant that belongs to the vitaceae family, its stem can climb up to a length of 30 foot, and it has five leaflet and round berries. The matured berries are bluish- purple in colour and it is one-seeded. They are found mostly in tropical Africa [13]. The leaves are well known for its nutritional and medical properties [14]. The seeds are discarded and little or no literature is available on its nutritive values and other uses of the seed. Therefore, this research work focused on the extraction of oil from the seed of cissus aralioides plant, and to further characterize and investigate the phytochemical content of the n –hexane extracted oil.
The matured barriers of Cissus aralioides was harvested from a farm located in Jos, Plateau state, Nigeria and identified by the department of Pharmacognosy, in University of Jos. The sample was washed and shade dried for three days to obtain the seeds, which were further dried for four days. Foreign materials were removed before milling. The milling machine was cleaned with ethanol and allowed to dry before milling into fine powder form.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 113
![]()
ISSN 2229-5518
Solvent extraction method was used to extract oil from cissus aralioides seeds, using n-hexane as the extracting solvent and Soxhlet extractor as the extracting medium.45g of the powdered sample was placed in thimble and 150ml of n- hexane was poured into a round bottom flask. It was heated at
650c for 10 hours. The experiment was repeated, using 50g of the sample and 150ml of n-hexane for 10 hours.
Saponification value (mg
KOH/g)
130.58 ± 0.30
The moisture content and characterization such as, percentage oil yield, cold test, relative density, refractive index, free fatty acid, acid value, iodine value, peroxide value and saponification value were determined respectively using standard procedure of the Association of Official Analytical Chemist [15]. GC-MS analysis was carried out according to standard methods [16].
The qualitative screening of bioactive component present in n- hexane extracted oil was was carried out using standard methods [16]. Qualitative test methods were employed, to evaluate for the presence of alkaloids, saponins, tannins, anthraquinones, carbohydrates, steroids, cardiac glycosides, and flavonoids. All tests were done in duplicates.
Table 1- Physico-Chemical Properties of Cissus Aralioides Seed
Oil
From table 1, the physical properties of the oil are the
yellowish colour and its stability as a liquid at room temperature. The moisture content is used to determine the stability and quality of the seeds. Studies has shown that plant with low moisture content have a longer shelf life [17]. The moisture content in the cissus aralioides seeds is moderately high with a value 25.1±0.18. As result of the moderately high moisture content, this may affect the stability and result to a shorter shelf life of the oil, since increase in moisture content result in an increase in peroxide value [18]. The percentage oil yield from cissus aralioides seed is 23.00 ±0.55. The yield is higher than the value reported in soybeans oil with value 18%, cotton seed oil with value 14% [19] and African star apple with value 21.57% [20]. The value is lower than the oil yield reported in palm oil, coconut oil and groundnut oil respectively [19] [21]. This may be because of the high moisture content, because for a good oil yield, the moisture content must be very low. The cold test is used to measure the resistance of oil to crystallization or turbidity. When Oil remains clear after cold test is described as salad oil . Cold test on cissus aralioides oil was observed to have low turbidity. Due to the presence of turbidity in the oil they cannot be described as salad oil and cannot withstand winterizing. Relative density of the oil obtained is 0.9220; this indicates that Cissus aralioides oil is less dense than water. And the value obtained is similar to that reported for rubber seed oil with value 0.9200 [22]. Refractive index is an indication of the level of unsaturation and chain length of oil. The refractive index at 250C of cissus aralioides oil is found to be 1.4758. The values obtained falls within the range of some common oils, with values between
1.3-1.5 [22] [23]. The acid value and free fatty acid value
obtained are 4.24 and 2.13± 0.025. This value is higher when compared with that of African star apple oil with value 4.50 and 2.25. The values are also lower when compared to that of groundnut oil with value 2.61 and 1.31 [23] [19]. The value obtained is an indication that the oil can easily go rancid. The iodine value which gives the degree of un-saturation of the oil was found to be 80.02g/100g. As a result of the value, the oil is classified as non-drying oil. Iodine value above 100 is classified as drying while those below are classified as non-drying [24]. The iodine value obtained is higher than the value reported for African star apple with value 35g/100g, also cashew nut oil with value 41.87±2.30g/100g [23] [25] but lower than groundnut oil with value 89.46g/100g, cotton oil with value
94.7g/100g [19] [26], due to its relative low iodine value, which results in classifying it as non-drying, it indicates that the oil
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 114
ISSN 2229-5518
has a low content of unsaturated fatty acids and can be employed in soap making and lubricant oil, which will help to reduce the dependence on edible oil in making such products. The peroxide value (PV) gives an idea of the level of rancidity in oil. The PV was found to be 34.67±0.10mEq.O2 kg-1. This value is higher when compared to the values obtained in groundnut oil with value 22.25 mEq.O2 kg-1 and African nut- meg with value 4.13±0.40 [19][27]. Studies has shown that there is a relationship between moisture content and peroxide value; increase in moisture content result in an increase in peroxide value [16]. From the PV obtained the high peroxide value maybe as a result of high moisture content in the seeds, this is an indication of the oil to get rancid easily, during processing or storage. The saponification value which gives an idea of the approximate chain length of the oil, and was found to be
137±0.30mg KOHg-1. This value is lower than the value reported in cashew nut oil with value 146±0.57 KOHg-1, cotton seed oil with value 189 KOHg-1 and groundnut oil with value
148.67 KOHg-1 [25][226][19] but higher than Jojoba seed oil with value 88 KOHg-1 . Jojoba seed oil is use in liquid soap making, antifoaming and shampoo [26]. This indicates that the oil could be used in soap making, and shampoo, since its saponification value falls within the range of these oils. It has been reported by that, the saponification value and molecular weight has an inverse relationship [27]; this means that, the shorter the saponification value the higher the molecular weight and the longer its’ chain length. The saponification value obtained which are moderately low, shows that it has a high molecular weight and long chain length. Figure 1, presents chromatogram and spectrum of Cissus aralioides sample oil.
SPECTRUM
![]()
![]()
Spectra match
Spectra match![]()
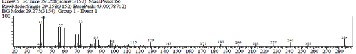
Spectra match![]()
Fig 1:Chromatogram and spectrum of cissus aralioides seed oil
Three of the spectra matched with the spectrum given from the library. They are, Diethyl Phthalate; 6-Octadecenoic acid and Octadecanoic acid, 2-(2-hydroxyethoxy) ethyl ester. Diethyl phthalate has been found in Citrus unshiu essential oil and Sansevieria Roxburghiana extract and it’s reported to inhibit Escherichia coli and Pseudomonas aeruginosa [28] [29]. 6- octadecenoic acid is a fatty acid that is classified as mono- saturated omega-12 fatty acid; this class of fatty acid is not common when compared to other classes such as ω−3, ω−6, ω−9. Other Studies reveal that ω−12 exhibited antimicrobial activity and are commonly use in cosmetic industries [30]. Octadecanoic,2-(2-hydroxyethoxy)ethyl ester found in this plant oil is also known as Diethylene glycol stearate and Aqua Cera (from the spectra library). This compound is used in cosmetic and textile industries. They serve as plasticizer, lubricant, binding and thickening agent [31].
Table 2, shows the phytochemical screening of oil from cissus aralioides seed, this revealed the presence of secondary metabolite which are alkaloids and steroids. While anthraquinones, carbohydrate, cardiac glycoside, flavonoids, saponins, and tannins were absent. The alkaloids were found to be slightly present, though plant alkaloids are said to active against bacterial and they are also used in treatment of skin disorders such as eczema, seborrheic dermatitis, and neurodermatitis [32]. Oil from cissus aralioides seeds also showed that steroids are largely present. Plant steroids are used in the cosmetic, soap and pharmaceutical industries to produces creams, soaps and ointments. They served as treatment against inflammatory diseases [33].
4.0 C ON C LU SION
![]()
In view of the results obtained from the various tests and analysis carried out on cissus aralioides seed oil, it showed
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 115
ISSN 2229-5518
that oil can be extracted from the cissus aralioides seeds, and it is a non-drying oil which have a short shelf life and easily become rancid based on the value obtained from its iodine value, moisture content and peroxide value (physio-chemical properties). It further revealed that the oil extract has a potential source of bioactive components, such as Alkaloids, and Steroids. The plant oil may be useful for antimicrobial, anti-bacterial and anti-inflammatory in pharmaceutical and medicinal industry. Also has its potentiality in soaps, cosmetic and pharmaceutical industries.
[1] W. Zheng, S.Y. Wang, “Antioxidant activity and Phenolic compounds in selected herbs,” Journal of Agriculture and Food Chemistry, vol. 49. no.11, pp. S157 –S170, 2001.
[2] G. Vannice, and H. Rasmussen. Position of the Academy of
Nutrition and Dietetics: “Dietary Fatty Acids for Healthy Adults”, Journal of the Academy of Nutrition and Dietetics, vol. 114, no. 1, pp.136-153, 2014
[3] U.Thiyam-Holländer, N. M. Eskin, and B.Matthäus, (Eds).
Canola. and Rapeseed: Production, Processing, Food Quality, and Nutrition. (CRC Press, 2012), pp.329-332.
[4] J. Bernal, J.A. Mendiola, E. Ibáñez, and A. Cifuentes, Advanced Analysis of Nutraceuticals. Journal of pharmaceutical and biomedical analysis, vol. 55, no. (4), pp. 758-774, 2011.
[5] J.P. Montmayeur, and J. Le Coutre, Fat detection: Taste,
texture, and post ingestive effects. Vol. 11 (CRC Press,
2010), pp. 265-269.
[6] C. Bingcan, D. McClements, and E. Decker, “Design of foods with bioactive lipids for improved health”, Annual review of Food science and Technology, vol.4, pp. 35- 36, 2013.
[7] B. Ajiboye, A. Oso, and O. Kobomoje, “Chemical
Composition and Nutritional Evaluation of Leea Guineensis Seed”, International Journal of Food Science, Nutrition and Dietetics, vol. 3, no.2, pp. 1 – 7, 2014.
[8] T. Win, “Oleic acid-The anti-breast cancer component in olive oil”. AU Journal of Technology, vol. 9, no. 1, pp. 75 –
78, 2005.
[9] O. Modupe, O. Wesley, A. Morufu, and A. Elizabeth, “Analysis of essential oil from the stem of Chansmanthera dependens”, Journal of Natural products, vol. 3, pp. 47-53,
2010.
[10] A. Warra, R. Umar, F. Atiku, A. Nasiru, and M. Gafar, “Physical and Phytochemical Characteristics of seed Oils
from Selected Cultivars Grown in Northern Nigeria”, Research and Reviews, Journal of Agriculture and Allied Sciences, vol.1, pp. 4 – 8, 2012.
[11] C. M. Hasler, J. B. Blumberg, “Symposium on Phytochemicals: Biochemistry and Physiology”, Journal of Nutrition, vol.129, pp. 756S-757S.
[12] R. Narasinga, “Bioactive phytochemicals in Indian foods
and their potential in health promotion and disease prevention. Asia Pacific Journal of Clinical Nutrition, vol. 12, no.1, pp. 9-22, 2012.
[13] F. Bongers, M. Parren, and D. Traore, Forest climbing plants of West Africa: diversity, ecology and management. CABI,
2005.
[14] C. Assob, L. Henri, S. Dickson, L. Anna, N. Peter, A.
Emmanuel, N. Abdel, S. Bertrand and B. Veronique,. “Antimicrobial and Toxicological Activities of Five Medicinal Plant Species from Cameroon Traditional Medicine”, BMC Complementary and Alternative Medicine, vol. 11, no. 70, pp. 1-11, 2011.
[15] AOAC International. Official methods of analysis (18th edn.). Association of Official Analytical Chemist, International, Gaithersburg, MD, 2007.
[16] R. Amina, B. Aliero, and M. Gumi, “Phytochemical Screening and Oil Yield of a Potential Herb, Camel Grass” Cent. Euro. Journal of Experimental Biology. Vol. 2, no.3, pp. 15b- 19, 2013.
[17] Y. Pomeranz, and C. Meloan, Food analysis: Theory and
Practice (Springer 2000)
[18] R. Akinoso, S. Aboaba, and T. Olayanju, “Effects of Moisture Content and Heat Treatment on Peroxide Value and Oxidative Stability of Un-Refined Sesame Oil” African Journal of Food Agriculture Nutrition and Development, vol.
10, no.10, pp. 4268-4285, 2010.
[19] Gunstone, Frank. (ed.), Vegetable oils in food technology:
composition, properties and uses (John Wiley & Sons, 2011. [20] K. Audu, E. Aluyor, S. Egualeona, and S. Momoh,
“Extraction and Characterization of Chrysophyllum albidum and Luffa cylindrica Seed oils”, Petroleum Technology Development Journal, vol.3, no. 1, pp. 1 – 7, 2013.
[21] N. Cynthia, O. Chukwuma, O. Benedict and U. Akuzuo, “Comparative Study of the Physicochemical Characterization of Some Oils as Potential Feedstock for Biodiesel Production”, International Scholarly Research Network, 2012.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 116
ISSN 2229-5518
[22] J. Asuquo, I. Anusiem. and E. Etim, “Extraction and Characterization of Rubber Seed Oil”, International Journal of Modern Chemistry, vol. 1, no.3, pp. 109 – 115, 2012.
[23] S. Adebayo, B. Orhevba, J. Musa, and O. Fase, “Solvent Extraction and Characterization of Oil from African Star Apple (Chrysophyllum Albidum) Seeds”, Academic Research International, vol. 3, no.2, pp. 178 -183, 2012.
[24] K. Julius, N. Umenger , and I. Ayangealumun, Effects of extraction Methods on the Yield and Quality Characteristics of Oils from Shea Nut. Journal of Food Resource Science, vol. 2, no. 1, pp. 1 -12, 2013.
[25] N. Idowu, and A. Abdulhamid, “Physicochemical and
Anti-Nutritional Factors Evaluation of Cashew (Anacardium Occidentale) Seed Nut Oil”, Advances in Agriculture, Sciences and Engineering Research, vol. 3, no.10, pp. 1205 – 1209, 2013.
[26] B. Orhevba, and A. Efomah, “Extraction and
characterization of cottonseed (gossypium) oil”, International Journal of Basic and Applied Science, vol.1, no.2, pp. 398-402, 2012.
[27] C. R., Ekeanyanwu, I. G.Ogu, and Nwachukwu U.,
“Biochemical characteristics of the African nutmeg
(monodora myristica)” Agricultural Journal, vol. 5, no. 5, pp.
303-308,. 2012
[28] Zaher A. F., Omayma S. El-Kinawy, J., and Dalia, E., “Solvent extraction of Jojoba oil from pre-pressed Jojoba meal ”, Grasas y Aceites, vol.55, no.2, pp. 129-134, 2004.
[29] B. Etuk , D. Udiong, and E. Akpakpan, The Effect of Lime on Some Physicochemical Properties of Palm Oil. International Journal of Modern Chemistry, 2(1), pp. 1 – 6,
2012.
[30] X. Yang, and S. Kang, “Chemical composition, antioxidant and antibacterial Activities of essential oil from Korean Citrus unshiu peel”, Journal of Agricultural Chemistry and Environment, vol.2, no.3, pp.42 – 49, 2013.
[31] D. Philip., P. Kaleena, and K. Valivittan, “GC-MS Analysis
and Antibacterial Activity of Chromatographically Separated Pure Fractions of Leaves of Sansevieria Roxburghiana”, Asian journal of pharmaceutical and clinical research, vol.4, no.4, pp. 130-133, 2011.
[32] V. Parthasarathy, B. Chempakam, and T. Zachariah.T. J.
(Eds.). Chemistry of Spices, CABI, 2008.
[33] G. Masesh , S. Ramkanth, and M. Saleem, “Anti- inflammatory drugs from medicinal plant- A comprehensive Review”, International Journal of Review in life Sciences. Vol. 1, no.1, pp. 1-11, 2011.
IJSER © 2015 http://www.ijser.org