2 litres flow for 8 minutes
Volume of filter water = 572.63cm2 x
14cm
= 8016.8cm3
Rate of flow = 8016.8cm3/8 minutes
= 1002.1cm3/minutes
¹Mechanical Engineering Department,
LadokeAkintola University ofTechnology,Ogbomoso, PMB 4000, OyoState, Nigeria.
²Mechanical Engineering Department, University of Agriculture, Abeokuta, Ogun State, Nigeria.
aaadekunle@lautech.edu.ng
Water is described as a universal solvent which is the most abundant and useful compound that nature has provided. Two main sources of water are: surface and underground water. Among the many essential elements for the existence of human beings, animal and plants, water is rated to be one of the most important elements for human living. Man can survive for days without food but not long enough without water.
Sand has been used to purify water for over a thousand years; and it still remains the dependable methods of making water fit for drinking. The idea of water sand filtration can be seen when water taken from sandy river beds is generally pure, because it has percolated through the sand grains where the bacteria harmful had to be eliminated.
As a result of high demand for quality and clean water by the society, various means to meet this demand has been earlier constructed. Though, all these means are not easily accessible by some of the community, due to unavailability and high cost, also, complexity of usage and lots more. This led to the design and construction of water filter which can be accessible by the community, this is of the significant that water filter is to be made available for the society.
Data gotten from the laboratory results clearly shows that an appreciable degree of treatment had taken place when the designed filter was used.
The problem of supplying adequate amount of water for distribution to the public is not limited to the construction of water works. Many water borne diseases have been traced to the defect in the operating procedure used in water treatment. Any untreated water contains active plant, animal and bacteria. All of these bacteria are harmful and are the pathogenic faecal bacterial which poses the greatest health hazard and this can be detected by the presence of Escherichia Coli (E. coli).
Filtration is the process by which water is purified by passing it through a porous medium. In slow sand filtration, a bed of fine
sand is used through which water percolate downward. Due to the fine grain size, the pore of the filter bed is small. The suspended matter present in the raw water is largely retained in the 0.5 – 2 cm part of the filter bed through electrostatic process. This allows the filter to be cleaned by scraping away the top layer of sand or by flushing. The filter cleaning operation need not to take more than a day but after cleaning, one or two more days are required for the filter bed to again become fully effective. A simple water filter working on the principle of slow sand filtration was improved upon. The filtration water treatment involves physical, chemical and biological changes that transform raw water into safe as specified by international standards for drinking water (WHO).
Literature Review
In 1980, it was declared that 1981 to 1990 should be the International Drinking Water Supply and sanitation’ by World Health organization. It was described by International Reference centre for community Water supply and Sanitation that slow sand filtration is one of the most suitable for the removal of pathogenic organisms (IRC, 2001).
The process of supplying of water to Amsterdam, Holland is purified simply by passing it through the ridges of sand on the coast. It is only essential that the sand is not allowed to dry so that the active ingredients in it will not depreciate in value. Any environment must be clean in order to protect their water from any germ. (Anderson, 2003).
Ogedengbe, 1985, stated that some particles can be grounded and heated to eliminate bulk of the volatile materials and then activated to develop proper pore Structure for absorption. He stated that the absorbent promotes sedimentation and absorption process during water filtration. In 1988 the principles behind slow sand filtration through electrostatic attraction has the most effective, but it occurs only between particles having opposite electrical charges. It is this that removes bacteriological content and does not remain there unchanged; it is transformed by bio- chemical and bacterial activity (Hofkes, 1988).
Ezeoma, 1988, also claimed that Ostreyko which was noted in 1980 that active absorbents could be obtained by subjecting carbonaceous materials as coconut shells and
strong fruit Kernels to some specific heat treatment.
Purpose of Water Treatment
As essential as water is to life, yet it could be dangerous if not safe, hence, need of water treatment must be provided to render it safe for drinking and domestic use. Most importantly is the removal of pathogenic organisms and toxic substances such as heavy metals causing health hazards, other substances may also be removed or at least considerably reduced. These include suspended matter causing turbidity, odour, colour, hardness and excessive carbon-di- oxide, corroding concrete and metal parts.
Many water – borne diseases have been traced to defect in the operating procedure used in water treatment plants (Camp. 1999).
Wagner and Lanoix reported that in the past, there had been plenty of outbreak of typhoid fever, cholera, guinea worm and epidemic jaundice due to breakdown of treatment. Water is very vital in the environment and it is very important that it should be treated before use (Charles, 2001).
Even in United State, twenty per cent of water- borne disease causes have been attributed to deficiencies in the operation of treatment plants. With treated water, the risk of water- borne disease is greatly reduced if not totally eliminated. Various treatment processes have been developed. Some serve as a single purpose while other serves multiple purposes. These processes include aeration, coagulation and flocculation, sedimentation, filtration (it may include slow sand, rapid sand and pressure filtration) and disinfection. (Craun and McCabe, 1973)
Important Uses Of Water
also used as coolant in power and chemical plant. In the areas of atomic energy and steel production, water is widely used. The construction industry finds water vital in forming concrete motors slaking lining and other structural materials.
1998).
Standards of Water Quality
Various laboratory tests which involve both physical and laboratory test were carried out on different Samples of water and Compared to standard set down by International Reference centre for Community Water Supply and Sanitation (IRC, 2001). It was stated by IRC that the levels of coliforms present in acceptable drinking water should be less than
10 per 100ml sample; and the number of
faecal E. Coli should be less than 2.5 per
100ml sample.
Further guidelines for quality of drinking water standards for community water supplies: are presented below. The World Health Organization established minimum criteria for drinking water (WHO, 1998).
Disease | Pathogen |
Typhoid | S. Typhi |
Bacterial Dysentery | ShigellaSp Campylobacter Sp E. coli |
Cholera | Vibrio Cholerae |
Amoebic Dysentery | E. Histotytica |
Dracotiasis | D. Medinensis (guinea worm) |
Schistosomiasis | S. Mansoni |
Schistosomiasis | S. Japanicum |
Schistosomiasis | S. heamatobuim |
Gastroenteritis | Rotaviruses |
Paratyphoid | S. Paratyphi |
Source: Medical laboratory Manual for Tropical Countries by MonicalCheesbrough
Water Quality Parameter | Measured as | Highest Desirable Level | Maximum Permissible Level |
Total dissolved solids | mg/1 | 500 | 2000 |
Turbidity | FTU | 5 | 25 |
Colour | mgpt/1 | |5 | 50 |
Iron | mgFe+/1 | 0.1 | 1.0 |
Manganese | ++ mgMn /1 | 0.05 | 0.5 |
Nitrate | mg No3/l | 50 | 100 |
Nitrate | mgN/1 | 1 | 2 |
Sulphate | mg5042-/l | 200 | 400 |
Fluride | mgF-/l | 1.0 | 2.0 |
Sodium | mgNa+/I | 120 | 400 |
Arsenic | mgAS+/1 | 0.05 | 0.1 |
Chromium (hexavalent) | Mgcr6+/1 | 0.05 | 0.1 |
Cyanide (free) | mgcN+/1 | 0.l | 0.2 |
Lead | mgPb/1 | 0.05 | 0.10 |
Mercury | NgHg/1 | 0.001 | 0.005 |
Cadmium | Mgcd/1 | 0.005 | 0.0100 |
Taste | - | ||
Chloride | CL/1 | 200 | 600 |
Zinc | Zn/1 | 5 | 15 |
Calcium | Cl/1 | 75 | 200 |
Magnesium | Mg/1 | 30 | 150 |
Sulphate | S03/1 | 200 | 400 |
Total hardness | Caco/1 | 100 | 500 |
Nitrate | No3/1 | 45 | - |
PH Value | PH metre | 7-8 | 6.5-9 |
Source: World Health Organization (1998)
Conventional Water Treatment Process
This involves settling and removal of suspended particles that take place when water still in, or flows through a filtering medium.It is a gravitational process where heavier particles than water are settled down; it is the most widely used operation. When the impurities are settled down by gravitational and natural segregation process, the operation is called Open Sedimentation. When a chemical is added to catalyse aggregation and settling of finely divided suspended matter, colloidal substances, and large molecules, the operation is called coagulation (Charles,
2001).
Coagulation and Flocculation provide the water treatment by which finely divided and suspended and colloidal matter in the water is made to agglomerate and form floes. Colloids are ‘stabilized’ (kept in suspension) by electrostatic repulsion and dehydration.
Common coagulants are Aluminium Sulphate (alum); ferric chloride (iron salt); Silicon, Bentonite etc. However, alum is by far the most widely used coagulants. (IRC, 2001).
Filtration is a process used in separation to remove impurities from drinking water by passing the raw water through a selective medium called Filter or Septum. Filter media are medium through which there is a selective passage of materials. Filtration helps in removal of pathogenic organisms from water; most especially the bacteria and viruses responsible water related diseases. In addition to this, control of colour, PH and turbidity are also affected by suitable filtration methods. (Jackson, 2000).
media. Filtration rate is h Rapid Sand Filtration does not given high bacteriologically safe drinking water since fast filtration process takes place which does not allow much settlement of impurities..
Slow sand filtration is highly efficient in reducing the total bacteria content by a factor of 1,000 to 10,000 and the E. Coli content by a factor of 100-1,000. A well operated slow sand filter will remove protozoa such as Endamoeba histolytic and helminths such as schistosomaheamatobium and Ascarislumbricoides. Trouble – free operation is possible when the average turbidity of the raw water is less than 5 F.T.U, with peak values below 20 F.T.U and occurring for periods of a few days only (Hofhes, 1981).
The theory behind slow sand filtration is based on electrostatic attraction and it occurs only between particles having opposite electrical charges. Clean quartz sand has a negative charge and is, therefore, unable to absorb negative charged particles such as bacteria, colloidal matter of organic origin, anions of nitrate, phosphate, and similar chemical compounds. During ripening period of a slow sand filter, only positive - charged particles are adsorbed, such as: carbonates, iron and aluminium hydroxide and cat ions of iron and manganese. Adsorption of positive charged particles will continue to a stage that over-saturation occurs. The overall charge of the filter bed grain coatings then reverses and become positive, after which negative-charged particles will be attracted and retained. After the initial ripening period of nitre bed will exhibit a varied and continuously varying series of negative and positive charged n
coatings that are able to adsorb most impurities from passing water. The matter accumulates on the filter sand grains does not remain there unchanged, it is transformed by bio-Chemical and bacterial activity. Soluble ferrous and magnesium compounds are turned into insoluble ferric and manganese oxide hydrates that become part of the coating around the sand grain. Organic matter is partly oxidised thus providing the energy needed by the bacteria for their metabolism (HOFKES,
1981).
Alternative Filtration Techniques
Filtration as techniques of separation has a wide application. Different liquid- solid separation devices make use of separating filtration processes.
Okpobisi, 1998, described filtration as process which involves impure water passing through beds of fine sand, coarse sand and large granular materials. As water percolates through these beds un-dissolved particles are removed.
Gesner G. Hawley, 1971, defined filtration as the process of separating suspended solids from a liquid by ‘forcing’ the mixture through a porous barrier.
From operation and controls of water treatment processes there are four types of water filters, which one sufficiently different to require separate discussion. It is essential that the selection of any one type be based on consideration of local factors as well as the advantages and disadvantages of each type. For economic and suitability purpose the porous barriers one of various types and particular ones are chosen after taken into consideration the nature of constituents to be filtered and other pertinent factor.( Charles R. Cox, 1971)
Various Filter Media And Their Uses
There are different filter media used for filtration. Filter media are the various medium through which selective passage of materials takes place.
Filter | Uses |
1. Metal fabrics/Screens | For handling toxic materials |
2. Pressed felts and cotton | Used to filter gelatinous materials paints |
3. Non-Woven fabrics (Polyester, nylon) | Used for gravity separation of oils |
4. Rigid porous media (e.g graphite, Sintered 1 painless steel) | Used for clarification |
5. Goths for Coarse fabric | Use in pressure filters and fruit juice Filtration. |
6. Thick sludges/slurres | Used in Vacuum or suction filters |
7. Ceramic membrane (Mainly alumina) | Used in water treatment plants |
8. Sand and gravel | Used in gravity filters |
9. Diatomaceous earth filters | In clarification of liquids in water of low turbidity |
10. Micro strainers | Used when plankton is present |
Source: Charles R. COX - Operation and control of water treatment, (1973).
Features And Applications Of Some Water
Filters
Water filters are devices used to purify water or reconditioning devices that make water tobe suitable for drinking, fire control, also in engineering purposes and industrial use as in boiler and other plants (chemical processing journal)
Various filters used in water treatment which are in existence are:-
(a) Slow sand filters
(b) Rapid sand filters
(c) Presses filters (i) Chamber press
(ii) Plate and frame press.
(d) Pressure leaf filters (i) Horizontal type
(ii) Vertical type
(e) Top outlet tubular filters
(f) Micro strainer
(g) Reverse Osmosis Filters.
Taking into consideration the objectives of this researchwork, and comparative analysis of various water filters in existence. Five water filters were selected and
studied closely.
Kind of Filters
Rapid Sand Filters
These filters are designed to operate at much higher rate than slow sand filter. Another name of it is “mechanical filter”, because the original units had mechanically
operated rakes to assist in agitating the sand during the washing process. Water flows through the sand by gravity.
The various units of rapid sand filter should be designed so as to be co-ordinated into whole plant in accordance with the basic plan. It is essential for the character of the water, the proposed rate of filtration, the size of the plant and the anticipated reliability of the operators to be considered in relationship to one another.
Specifications of the filter bed for rapid sand should be selected with care, with due regard to the anticipated effectiveness of pre- treatment, the rate of filtration and the depth of sand. When unit rate of filtration is 4.8m/h, the sand effective size is in the range of 0.40 -
0.50mm to secure reliable filtration. For higher
rates of filtration, the size should be between
0.50 and 0.7mm. A factor of safety is provided by a sand depth of 0.75-0.90m. The minimum depth should not be less than 0.6m, (Rox.
1960)
The normal rate of filtration is 80mm/min of filter area. Rates range from 40 to 200 mm/min. The lower rates provide factors of safety but naturally results in need for larger filters.
(i) Its construction is simple
(ii) The flowing rate of filtration is generally high, between 40 and 200 mm/min.
(iii) It is useful in treatment of very turbid river water
(i) It is not as efficient as slow sand filter in the removal of pathogenic organism e.g bacteria, and viruses.
(ii) It is more difficult in maintenances compare to slow sand filter because backwashing is necessary for clearing.
Choosing The Most Appropriate Filter
Having considered different kinds of filters, it was considered that slow sand filtration method of filtering is the most appropriate type for this research work. This can be deduced from the following reasons:
i. It has the highest efficient means of removal of pathogenic organisms that is water related diseases.
ii. It is the most useful means of removing suspended particles by
coagulation and flocculation.
iii. It is cheapest in construction
iv. Materials employed are locally gotten.
v. Easy to operate and produced by rural house wives and urban dwellers.
vi. Maintenance is easier through
backwashing method
vii. Materials used are not susceptible to corrosion of chemical processes of water.
This research is incomplete without saying that, though the slow sand filtration has some limitations, but the efficiency surpassed these limitations,
this explains why it is said to be the best method of water filtration.
Principle Of Slow Sand Filtration
In slow sand filtration, the removal of impurities from the raw water is brought about by a combination of different processes such as
sedimentation, adsorption, straining and; most importantly, biochemical and microbial actions. (Hofkes, 1981)
Filtration is the process whereby water is purified by passing it through beds of sand which contains slime organisms on the sand grain. Absorption is the removal of suspended solids, together with colloidal and dissolved impurities around the filter bed grains or through physical mass attraction and electrostatic attraction (IRC, 2001)
In electrostatic attraction, particles with opposite electrical charges are absorbed. Quartz sand being negatively charged removes flock of
carbonates, iron and aluminium hydroxide as well as cat ion of iron and Manganese. Reversal of overall charges results in removal of positively charged particles such as bacterial, colloidal matter of organic origins, anions of nitrate phosphate and other similar chemical compounds. Addition of activated charcoal to the top layers of the bed is an improvement in the absorption process, lowering of turbidity, increase coagulation; flocculation, purifying acids, recovering solvents and decolourizing of liquids. It must be able to wet easily.
The activity of Biochemical is useful in
meaningful method of reducing morbidity due to enteric disease which is becoming widely acknowledged. Previous studies have established that the entire diseases caused by intestinal bacteria are caused exclusively by the faecal contamination of water and food materials (Otunola, 1980).
Design Of Filter Tank
For the actual design of a slow sand filter tank, four dimensions have to be chosen in advances; the depth of the
filter bed, the grain size distribution of the filter material, the rate of filtration, and the depth of supernatant water (IRC, 2001)
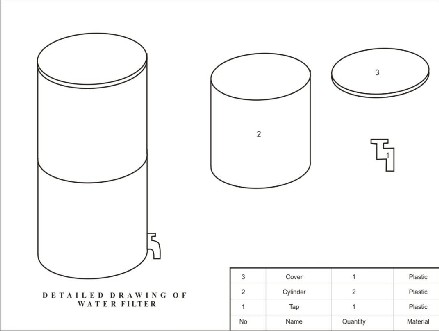
The filter tank is a cylindrical like sheet of height 27cm by 27cm diameter. It
has a cover which can be removed to
pour in raw water. The tank is divided
into two parts; the upper part helps in spreading raw water over the filter bed. The bottom screen is to hold the filter media at the base of the tank and filtered water passes through it. The bottom screen is 26cm from the base,
the distance which serves as reservoir for the filtrate.
Design Of Filter Media
A mixed filter media of different sizes of sands will be arranged in order below:
(a) Activated charcoals of roughly
0.01 - 0.02mm
(b) Fine sand of effective grain size 0.02 - 0.05mm
(c) Coarse sand of effective grain size 0.02 - 0.05mm
(d) Activated carbon
(e) Small size gravel of effective grain size 1.5 - 2.5mm
(f) Big size gravel of effective grain size 2.6 - 30mm
Arrangement is from top to bottom in order a bcdef. Total layers is of depth 10.5cm.
part, which contains the filter media, was filled with different grades of sand and gravel. A tap was fitted to the lower part of the filter to receive the filtered water.
Capacity Of The Tank
Following the design model for the filter bed:
Length of bed 10.5cm
Diameter of the bed, d, = 27cm
Length of water space above beds =
14cm
The cross sectional area of the filter bed can be gotten from ∆A =π/4 * d2
Now,
Area of water flow = π/4 * 272
=572.63cm2
1 litres = 1000cm3
35 litres = 35000cm3
Now,
Construction Of Tank
Plastic container of equal cross- sectional area was used; the diameter of the container is 27cm. The top part is fitted such that it can be removed for maintenance. The perforated upper
2 litres flow for 8 minutes
Volume of filter water = 572.63cm2 x
14cm
= 8016.8cm3
Rate of flow = 8016.8cm3/8 minutes
= 1002.1cm3/minutes
Maintenance Of The Filter
The bed of the filter was designed for the use of local house wives and the children who require simplicity. Operating the filter for 18 hours is also
efficient and prolongs its life span. In situation that the filter is to be changed, the following steps are useful.
(A) Top part should be removed
and wash.
(B) Layers of filter bed are removed gently.
(C) The bottom part is washed.
(D) New layers of clean sand and gravel are re-arranged and place activated charcoal on the filter bed.
Results obtained from the laboratory examination of the raw water are outlined below.
Grain Stain | Spore Staining | Motility | Microscope | |
E.Coli | Nil | Light green | Nil | Light Stain |
CHARACTERISTICS | RAW VALUE |
Colour | 42 mgpt/l |
Temperature | 300C |
7.9 | |
pH | 7.9 |
84 mg/l | |
Total Hardness | 84 mg/l |
48 mg/l | |
Calcium Hardness | 48 mg/l |
21.30 mg/l | |
Calcium ion | 21.30 mg/l |
49 mg/l | |
Chloride ion | 49 mg/l |
31.20 mg/l | |
Magnesium ion | 31.20 mg/l |
0.4 | |
Iron | 0.4 |
60 mg/l | |
Coagulation | 60 mg/l |
200 mg/l | |
Calcium Carbonate | 200 mg/l |
When the result of the raw water was compared with the standard WHO, given data, the result shows that the raw water was not suitable for drinking. There was still need for an improvement in parameters of water, in
addition to an improvement in bacteriological content. For the filter to be efficient, it must be able to bring about treatment that will render the water fit for drinking.
Treated Water Analysis.
When the filtrate collected from the designed filter was analysed, the following results was gotten.
CHARACTERISTICS | RAW VALUE |
Colour | 8 mg/pt/l |
Temperature | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
pH | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Total Hardness | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Calcium Hardness | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Calcium ion | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Chloride ion | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Magnesium ion | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Iron | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Coagulation | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Calcium Carbonate | 300C 7.3 73 mg/l 40 mg/l 15 mg/l 20.0 mg/l 15.23 mg/l 0.13 20 mg/l 96 mg/l |
Grain Stain | Spore Staining | Motility | Test | Microscope | |
E.Coli | Nil | Nil | Nil | Absent |
Operation Of The Tank
Water to be filtered will be poured through the top screen which spreads it on the filter bed. Percolation will be coupled with some active slime ingredients present on the skin of the sands will bring about filtration. After some minutes filtered water will be collected inside the reservoir.
have laminar flow Conditions at any point in the fluid do not change with respect to time![]()
8t = 0 …….. 1
The French engineers Darcy Henry
(1803-58) published result from which he
deduced Darcy’s law. Darcy’s equation is given below![]()
V = ±C dx ……….2
Where:
V is steady mean velocity (m3m-2h-1)
It is a fact that, from laboratory results that an appreciable degree of treatment had tremendously been gotten when the designed filter was used.
1. From the data after treatment, the water becomes unobjectionable using the designed filter.
2. There is a tremendous elimination of
bacteria present in the water especially
Escheria Coli.
3. The PH value after using the filter was okay.
4. Rate of filtration is slower using the designed filter.
5. Absorption through electrostatics is higher and efficient with the filter.
Qualities Of Drinking Water
Potable water should be free from all pathogenic organisms, dissolved poisonous chemical substances of all kinds’ objectionable gases and dissolved minerals which impact excessive hardness to water.
It should be colourless and odourless, portability of water can be determined after analysing various qualities. The analysis includes chemical, bacteriological and microscopic parameter. For unused sources, the physical and chemical parameters could provide adequate information as to portability of water.(Holfkes, E. H. 2000).
Physical Parameters
The attractiveness of water to human senses is determined by physical parameters. Commonly physical parameters are colour, taste, turbidity, total solids, and temperature.
Colour is an appeal to sensation which is the true appearance of water sample. The colour has no sanitary significance. It must be unobjectionable (Girout, E. 1999).
Water odour and taste are caused by the presence of excessive and inorganic substance. They are said to be objectionable or unobjectionable as they have no influence to the passage of light through water. Turbidity of water is the measure of interference caused to the passage of light through water. Turbidity is not detrimental to water portability unless the turbid water contains diseases causing organism.
Pathogenic Parameters
Water may still contain large numbers of
bacterial and yet still be safe for drinking as long as pathogenic for human are lacking. Water from stream and lake which contain multitudes of autotrophs and saprophytic heterotrophs is potable as long as human pathogens are lacking (Ogundana, 1989)
Chemical Parameters
Analysing chemical parameters have wide application in determining the adequacy of water supplies.
Chemical parameters could be divided into
i. Toxic substances
ii. Substances which affect the acceptability of water.
iii. Substance which are hazardous to the health.
iv. Substance which indicate pollution.
Some substances when present in water affects its acceptability as a domestics supply, although it may sometimes not cause health hazards to the people consuming it. There are some that are hazardous to health, such as fluorides and nitrites; excess of fluorides may cause dental flourishes.
The following conclusions can be made from this research. The slow sand filtration method is the most suitable among several treatment processes, locally available materials were used in the construction.
Moreso, the depth and capacity of filter bed were increased which made it to be more efficient to an appreciable degree. In conclusion, despites the fact that water gotten from the tap has undergone some treatments, it still needs to be filtered for it to be safe for drinking. An efficient filter tank having more capacity using slow sand filtration method with inclusion of activated charcoal and the filter bed length increased has been produced.
1. Anderson P. (2003): “Environment Sanitation Manual”. Department of Aquatic Biology, Exeter University, England.
Handbook of Hydraulic” 2nd Edition; McGraw Hill Book Co. New York; pp 30
“Almumni Magazine”University of Calgary
Sanitation” Hague, Netherland. Pp 22-26.
6. Charles R.C. (1973) “Operation and Control of Water treatment” 3rd Edition; Geneva, World Health Organization Book; pp 30
7. Craun and McCabe, L. J (1973):“Review
Of The Causes Of Water Borne Diseases Outbreak” Journals of the American Water Works Association. pp74-84
Department of Chemical Engineering, OAU, Ile – ife.
Condensed Chemical Dictionary” Van Norstrand Reinhold Company, New York. pp 375
Borne Diseases” McGraw Hill Book Co. New York
Community Water Supplies Technology Of Small Water Supply System In Developing Countries”.Hague, Netherland
9 – 359
Disease” First Edition, Robert Hill Book
Co., New York. Pp 28-36.
Laboratory Manual for Tropical Countries”. Microbiology; Tropical Health Tchnology; Vol 11.Heinemun England.pp 212 -222
Microbiology” A Laboratory Manual, O. A. U. Press, Ile – Ife, Nigeria. pp 143 –
147
Civil Engineering Journal,Nigeria. May,pp 4-9.
Bacteriological Quality of Water” Biology Department, Kwara State Polytechnic.
Media Filtration with sand and palmKennel Shell”International Journal for development of technology vol 3, pp
251 – 260
Locally Available Materials For Water Treatment In Nigeria”B. Sc Thesis Submitted To Department Of Civil Engineering University Of Ibadan.
Supplies and Sanitation”Blair Research
Laboratory, Ministry of Health, Harare.
Water Quality”.Vol 1, WHO Hague
He is a member of Nigeria Society of Engineers, and his research interests are Production Engineering.