International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1226
ISSN 2229-5518
Biosynthesized Gold Nanoparticles as Catalyst
Swarnali Maiti, Gadadhar Barman and Jayasree Konar Laha*
Department of Chemistry, Midnapore College, Midnapore-721101, India
Abstract— Citrullus lanatus (watermelon) fruit juice is a delicious multivitamin drink of great medicinal significances. Nutritionally, Citrullus lanatus is a good source of vitamin A and C. W e have used this juice for the synthesis of gold nanoparticles (AuNP) under very gentle condition. The resulting AuNP has been used as a catalyst. The catalytic activity of the as-prepared gold nanoparticles was observed in the reduction reaction of 2, 4- Dinitrophenyl hydrazine to 2, 4-Diaminophenyl hydrazine in presence of NaBH 4 . The reduction progression with time has been monitored from the UV-Vis spectra. No change in absorption spectrum was observed on addition of freshly prepared aqueous solution of sodium borohydride in 2, 4-DNP solution. Reduction of the nitro group to amino group only took place when AuNP solution was added in the mixture and the reaction was very fast. A new peak due to the reduction product (amino analogue) developed immediately at 300 nm as soon as AuNP solution was added and the peak due to the 2, 4-DNP at 358 nm was abolished. The intensity of the new peak gradually increased with time. Complete elimination of 358nm peak and subsequent emergence of
300 nm peak within a few minutes indicated the catalytic activity of the AuNP solution.
Index Terms Green synthesis, Gold nanoparticle, Citrullus Lanatus, UV-Visible spectroscopy, Catalytic reduction, 2, 4-dinitrophenyl hydrazine, 2 ,4- diaminophenyl hydrazine.
—————————— ——————————
Nanotechnology is an emerging field of science which involves synthesis and applications of nanoparticles. Metal nanoparticles are extensively used in various applica- tions including electronics, bio sensing and surface enhanced Raman spectroscopy. During past two decades an enormous effort has been invested in the investigations of metal nano- particles because of their applications including as vehicles for drug delivery, catalysts, biotechnology, biodiagonstics biosen- sors, imaging and visualizing agents etc [1-6]. Among the var- ious applications catalysis at the nanoscale level has attracted significant attention in the past two decades due to the unique properties of materials that arise at that level [7]. Nanoparti- cles are important catalysts for pollutant removal [8], energy conversion [9, 10], etc. As compared to their bulk counterparts, nanoparticles are often superior or new catalytic properties result from their nanometer size, which gives them increased surface-to-volume and chemical potentials. The catalytic prop- erties of AuNP have been explored. Gold nano catalysts have found a wide range of applications including CO oxidation [11], formic acid electro oxidation [12], alcohol dehydrogena- tion [13], etc. Tsunoyama et al. have used colloidal AuNP as catalyst for carbon-carbon bond formation [14]. Sau et al. have used gold nanoparticles for the reduction of Eosin [15].
AuNP can be synthesized by conventional chemical
and physical methods [16-18]. Many of the synthetic routs for development of nanomaterials using of toxic reagents make
them unsuitable for biological use and also have adverse effect
on environment. So, development of green processes for the synthesis of nanoparticles has evolved into an important branch of nanotechnology. Green nanotechnology uses bio- molecules present either in plant extracts or micro-organism as novel reducing and capping agents. They not only suffice as environmentally friendly routs but also economically sustain- able alternatives to chemical and physical methods. Recently, many biosynthetic routs have been reported for the synthesis of AuNP through micro-organism such as algae, fungi and bacteria. However, due to their cumbersome procedure such as maintaining cell cultures under specified laboratory condi- tions have made them less attractive compared to plant prod- ucts for synthesis of nanomaterials. So, the synthesis of AuNP by using renewable plant or plant products [19-25] has re- ceived tremendous attention in recent years.
In our present work, the synthesis of gold nanoparti- cles has been carried out using the juice extract of Citrullus lanatus. Nutritionally, the Citrullus lanatus is a good source of vitamin A and C. The thiamine, riboflavin, niacin, carbohy- drates, lipids, proteins are the major polysaccharides present in it. Ascorbic acid is the most abundant organic acid with some pantothenic and folic acid [26]. Thus the Citrullus lanatus juice mostly contains proteins and water soluble organic acids. The presence of organic acids and polyphenols in the Citrullus lanatus juice may be responsible for the reduction of Au+3 to Au0 ions and stabilization of AuNP.
.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1227
ISSN 2229-5518
Chloroauric acid was of AR grade, purchased from Sigma-Aldrich Chemical Ltd. Sodium hydroxide and 2, 4- Dinitrophenylhydrazine (2, 4 DNP) and sodium borohydride were purchased from Merck. Double distilled de-ionized wa- ter was used in all experiments.
The Citrullus lanatus was collected from local market and washed with Double distilled de-ionized water. It was cut into pieces. The extract was made from the inner red portion of the fruit and it was filtered using whatman filter paper.
AuNP was produced by reduction of Chloroauric acid solu- tion using Citrullus lanatus juice extract. 5 ml of double dis- tilled deionized water was added to 5 ml of pure juice extract to make it 1:1 and it was cooled in ice cold water. The solution was made alkaline (pH 10) by adding NaOH. The whole appa- ratus was placed on a heating mantle. During heating 6 ml
3×10-3(M) aqueous chloroauric acid solution was added drop wise with continuous stirring from burette and finally it was heated further for 20 minutes at 700C. The color of the solution gradually changed from light pink to violet. The violet color indicated the formation of AuNP.
The absorbance spectra of the AuNP were taken by using a ‘SHIMADZU’ UV 1800 spectrophotometer and for TEM images JEOL-JEM 2100 high resolution transmission electron microscope (HR-TEM) was used. Samples for the TEM studies were prepared by placing a drop of the aqueous suspension of particles on carbon-coated copper grids fol- lowed by solvent evaporation under vacuum.
The catalytic activity of the as-prepared AuNP was examined by the reduction of 2, 4-DNP to 2, 4-Diaminophenylhydrazine in presence of NaBH4. 0.15 mM solution of 2, 4 DNP was pre-
• Swarnali Maiti is currently pursuing PhD degree pro- gram in Midnapore College, Vidyasagar University. In- dia, PH-09932666591. E-mail: maiti.sonali@gmail.com Gadadhar Barman is pursuing PhD degree program in Midnapore College, Vidyasagar University. India,E- mail:bgadadhar@rediffmail.com
pared in ethyl alcohol and it was diluted 10 times with dis-
tilled water. The catalytic reduction was conducted using this diluted 2, 4 DNP solution at room temperature. For reduction, freshly prepared 200 µl of 0.125 mM NaBH4 solution was add- ed in 4ml of 2, 4 DNP solution. No change was observed visu- ally as well as spectroscopically due to the addition of NaBH4 only which confirms the failure of reduction. But, immediate occurrence of reduction was observed in presence of 250 µl of as prepared AuNP in the mixture.
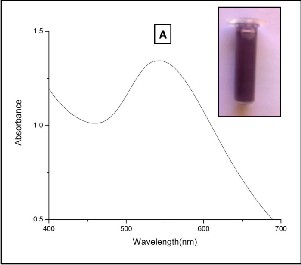
In our present study, a smooth and narrow absorption band was observed at 540 nm due to the formation of gold nanoparticles using 1:1 composition of Citrullus lanatus juice extract (Fig. 1A). The Citrullus lanatus juice mostly contained proteins and water soluble organic acids like ascorbic acid, amino acids and vitamins. We believe that the organic acids as well as polyphenols have the major involvement in reduction as well as stabilization of AuNP.
The morphologies and the orientations of nanoparti- cles are generally observed from the TEM studies. Fig.2. shows the TEM image of AuNP produced from 1:1 composi- tion of Citrullus lanatus extract. It is observed that the particles were mostly spherical and the hysteresis curve (Fig. 2C) for the particle size distribution confirmed that their sizes varied from 5 to 25 nm. Selected area diffraction pattern (SAED) illus- trated the crystalline nature of the AuNP (Fig. 2B.)
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1228
ISSN 2229-5518

FTIR analysis was performed to identify the bio- molecules localized on the surface and responsible for the re- duction of Chloroauric Acid. Representative FTIR spectra of pure Citrullus lanatus juice and the synthesized AuNP are shown in Fig. 3A and 3B respectively. The FTIR spectra before the bioreduction of Au+3 ions (Fig.3A) showed a broad spec- trum between 3300-3600 cm-1 due to hydroxyl (OH) stretching bands of polyphenols and also might be due to the N-H group of pteridine ring of folic acid, riboflavins, thiamine, niacin etc [27]. The absorbance peak around 2934 cm-1 indicated the presence of aromatic C-H stretching [28]. The peak at 2858 cm-
1indicated the C-H stretching of aliphatic amines and the aro-
matic C-H out of plane deformation band occured below700 cm-1. The absorbance peak at 1000-1200 cm-1 indicated C-O single bond and a peak at 1636 cm-1 indicated the presence of carbonyl group (C=O)form the polyphenols and organic acids such as folic acid, ascorbic acid. The absorbance peak at 1062 cm-1 corresponding to C-O stretching vibrations of alcohols, or phenols also confirmed the presence of polyphenols [29]. The broad spectrum at 3300-3600 cm-1 of pure Citrullus lanatus juice extract became slightly narrower and also slight change of band at 1636 cm-1 and disappearances of peak at1062cm-1 indi- cated the major involvement of polyphenols in the biosynthe- sis procedure.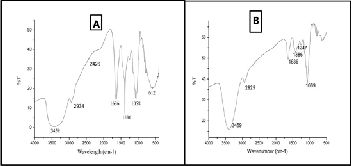
lus lanatus juice.
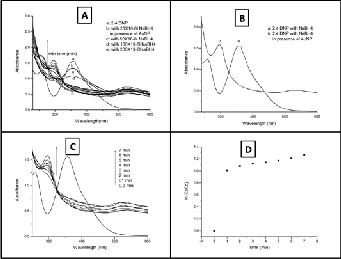
2, 4-DNP solution shows absorption maxima at 358 nm in UV-Visible spectrum. No change in absorption spec- trum was observed on addition of freshly prepared aqueous solution of sodium borohydride in 2, 4-DNP solution. The pic- ture did not change even keeping the solution for several days. Which suggests that the reduction of 2, 4-dinitrophenyl hy- drazine by sodium borohydride is kinetically not favourable though the reduction reaction being thermodynamically fa- vourable. Reduction of the nitro group to amino group only took place when AuNP solution was added and the reaction was very fast. A new peak due to the reduction product (ami- no analogue) developed immediately at 300 nm as soon as AuNP solution was added and the peak due to the 2, 4-DNP at
358 nm was abolished. The intensity of the new peak gradual- ly increased with time. Complete elimination of 358nm peak and subsequent emergence of 300 nm peak within a few minutes indicated the catalytic activity of the AuNP solution.
To optimize the reaction condition and to establish the
effects of AuNP and NaBH4 on the reduction process, the ex- periment was carried out several times by changing the con- centrations of both the precursors. The reaction was first car- ried out without adding NaBH4 in the solution mixture con- taining 2, 4 DNP and AuNP and stirred thoroughly. No peak due to the amino product generated but only two peaks were observed, one at 540 nm due to AuNP and another at 358 nm for 2, 4 DNP in the UV-Vis spectrum . So it confirms that AuNP itself is unable to reduce 2, 4 DNP. In an attempt to op- timize the amount of NaBH4 solution required to have reduc- tion in presence of AuNP, amount of NaBH4 was varied time to time. The experiment established that the minimum concen- tration of NaBH4 required for reduction reaction to occur for 4 ml mixture of 2, 4- DNP and AuNP solution was 200 µl. The reduction tenure was observed for 10 minutes. During the re- duction process the intensity of the peak due to the amino product increased appreciably. After 7 minutes of reaction time no further changes was observed. This indicated the completion of the reduction reaction. The relationship be- tween ln (Ct/Co) and time (Fig. 4D) revealed a linear correla- tion, where Co and Ct are the concentrations of 2, 4- diamino phenyl hydrazine at time 0 and time t, respectively. The ratio of the absorbance, At /Ao here has been substituted for the ra- tio of the concentration, Ct/Co because the concentration of it is proportional to its absorbance.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1229
ISSN 2229-5518
A very mild method for the synthesis of AuNP has been reported by using Citrullus lanatus juice, a well known multivitamin drink of great medicinal importance. The organic acids as well as polyphenols present in the juice ex- tract were responsible for the reduction and stabilization of gold nanoparticles. The resulting AuNP has been used as a catalyst. The catalytic activity of the as-prepared gold nano- particles was observed in the reduction reaction of 2, 4- Dini- trophenyl hydrazine to 2, 4-Diaminophenyl hydrazine in pres- ence of NaBH4 . No change in absorption spectrum was ob- served on addition of freshly prepared aqueous solution of sodium borohydride in 2, 4-DNP solution. Reduction of the nitro group to amino group only took place when AuNP solu- tion was added and the reaction was very fast. A new peak due to the reduction product (amino analogue) developed immediately at 300 nm as soon as AuNP solution was added and the peak due to the 2, 4-DNP at 358 nm was abolished. The intensity of the new peak gradually increased with time. After 7 minutes of reaction time no further changes was ob- served. This indicated the completion of the reduction reac- tion.
We are thankful to Central Research Facility at IIT
Kharagpur, India for HR-TEM measurements.
[1] E.C.Dreaden, L.A. Austin, M.A.Mackey and M.A. EL- Sayed,” Size matters: gold nanoparticles in targeted cancer drug delivery”. Ther Deliv, Vol 3, pp. 457-478,
2012
[2]L. Vigderman, E.R.Zubarev,” Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules”. Adv Drug Deliv Rev, Vol 65, pp.663-676,
2013
[3]S.A.Aromal, K.V.D.Babu, D.Philip.”Characterization and catalytic activity of gold nanoparticles synthesized using ayur- vedic arishtams”. Spectrochim. Acta A: Mol. Biomol. Vol
96, pp.1025-1030, 2012
[4] L.Dykman, N.Khlebtsov, “Gold nanoparticles in biomed- ical applications: recent advances and perspectives”, Chem. Soc. Rev. Vol 4 no.1, pp. 2256-2282, 2012
[5] A.J. Mieszawaska, W.J.Mulder, Z.A. Fayed,
D.P.Cormode, “Multifunctional gold nanoparticles for diag- nosis and therapy of disease”, Mol Pharm, Vol 10, pp. 831-
847, 2013
[6] S.R.Beerman, F.P.Zamborini, ”Purification of gold nano- plates grown directlyon surfaces for enhanced localized surface plasmon resonance biosensing”. ACS Nano, Vol 4, pp. 3633-
3646, 2010
[7] N.Toshima, T.Yonezawa,”Bimetallic nanoparticles- Nov- el Materials for Chemical and Physical Applications.” New J. Chem. Vol 22, pp. 1179-1201, 1998
[8]A.R. Khataee, M.B.Kasiri, L. Alidokht,” Application of response surface methodology in the optimization of photocata- lytic removal of environmental pollutants using nanocata- lysts”.Environmental Technology, Vol 32, no. 15, pp.1669-
1684, 2011
[9]V.L. Nguyen, Y. Yong, M. Cao, N. Masayuki,” the de- velopment of mixture alloy and core-shell nanocatalysts with nanomaterials supports for energy conversion in low tempera- ture fuel cells”.Nano Energy, Vol 2, no. 5 , pp. 636-676,
2013
[10]H. Zhiyu, B. Vassil, T. Thomas, “Nanocatalytic sponta- neous ignition and self- supporting Room Temperature Com- bustion”.Energy Fuels, Vol 19, no. 3, pp. 855-858, 2005
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1230
ISSN 2229-5518
[11]H. Ago, K. Murata, M. Yumura, J. Yotani, S. Uemura, “Ink –Jet printing of Nanoparticle Catalyst for Site- Selective Carbon Nanotube Growth”. Appl. Phys. Lett. Vol 82, 2003 [12]P. Waszczuk, T. M. Barnard, C. Rice, R.I.Masel, A. Wieckowski, “A Nanoparticle Catalyst with Superior Activi- ty for Electrooxidation of Formic Acid”. Electrochem. Com- mun.Vol 4, pp. 599-603, 2002
[13] T. Mitsudome, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa, K. Kaneda, “Oxidant free Alcohol Dehydrogena- tion Using a Resuable Hydrotalcite –supported Silver nanopar- ticle Catalyst”. Angew. Chem. Vol 120, pp. 144-147, 2008 [14]H. Tsunoyama, H.Sakurai, N.Ichikuni, Y.Negishi, T. Tsukuda, “Colloidal gold nanoparticles as catalyst for Carbon- Carbon bond formation: Application to Aerobic Homocoupling of Phenylboronic acid in water”, Langmuir, Vol 20 no. 26, pp.11293-11296. 2004
[15]T.K.Sau, A.Pal, T.S.al,” Size Regime Dependent catalysis by gold nanoparticles for the reduction of Eosin”, Vol 105 no.
38, pp. 9266-9272. 2001
[16] Z.Y.Chen, Y.Zhou, C.Y.Wang, Y.R. Zhu, “A novel ul- traviolet irradiation technique for shape –controlled synthesis of gold nanoparticles at room temperature”. Chem. Mater. Vol 11, pp. 2310-2312. 1999
[17] K.Esumi, A.Kameo, A. Suzuki, K, Torigoe, T. Yoshi- mura, Y.Koide, H. Shosenji, “Preparation of gold nanoparti- cles using 2- vinyl pyridine telomeres possessing multi- hydro- carbon chains as stabilizer”. Coll. Surf. A Vol 176, pp. 233-
239, 2003
[18] M.Y.Han, C.H.Quek, W.Huang, C.H.Chew, L.M. Gan, “A simple and effective chemical route for the preparation of uniform nonaqueous gold colloids”. Chem. Mater. Vol 11, pp. 1144-1147, 1999
[19]G.Barman, S. Maiti, J.Konar Laha,” Bio-fabrication of gold nanoparticles using aqueous extract of red tomato and its use as a colorimetric sensor”. Nanoscale Res. Lett. Vol 8, pp.181-189, 2013
[20] S.P.Chandran, M. Chaudhury, R.Pasricha, A.Ahmed, M. Sastry. “Synthesis of gold nanotriangles and silver nano- particles using Aloe vera plant extracts”. Biotechnol Prog, Vol 22, pp. 577-583, 2006
[21]J.L.Gardea- Torresdey, J.G.Persons, E. Gomez, Peral-
ta- J.Videa, H.E.Troiani, P.Santiago, M.J.Yacaman, “For- mation and growth of Au nanoparticles inside life Alfalfa
plants”. Nano Lett. Vol 2, pp. 397-401, 2002
[22] Daizy Philip. “Honey mediated green synthesis of gold nanoparticles”. Spectrochimica Acta A: Mol. and Biomol. Spectro. Vol 73, no.4, pp. 560-563. 2009
[23] R.K. Das, B.B. Borthakur, U. Bora,” Green synthesis of
gold nanoparticles using ethanolic leaf extract of Centella asiat- ica”. Mat. Lett. Vol 64, no.13, 1445-1447, 2010
[24] S.P. Dubey, M. Lahtinen, M. Sillanpaa.” Green synthe- sis and characterization of silver and gold nanoparticles using leaf extract of Rosa rogusa”. Colloid and Surf. A: physico- chemical and Engg. Aspects, Vol 364, pp. 34-41, 2010 [25]M. Naruzi, D. Zare, K. Khoshnevisan, D. Davoodi. : “Rapid green synthesis of gold nanoparticles using Rosa hy- brida petal extract at room temperature”. Spectrochim. Acta part A. Mol. and Biomol. Spectro. Vol 79, no. 5, 1461-
1465, 2011.
[26] USDA National Nutrient data base. Watermelon
(Citrullus Lanatus), fresh, Nutritive Value per 100 g. [27]T.S. Renugadevi, S. Gayathri, Int. J. pharm. Sci. Rev.
Res. Vol 2, pp.106, 2010
[28]G, Socrates, Infrared Characteistic Group Frequencies, Wiley, New York (1980)
[29]J.Y. Song, H.K. Jang, B.S. Kim,” Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts.” Process Biochem. Vol.44,no. 10 pp. 1133-
1138, 2009
IJSER © 2014 http://www.ijser.org