International Journal of Scientific & Engineering Research, Volume 3, Issue 7, July-2012 1
ISSN 2229-5518
Biosurfactant Production by Microorganism for Enhanced Oil Recovery
Elyas Golabi1, Seyed Ruhollah Mortazavi Poor Sogh1, Seyed Noroldin Hosseini1, Mohammad Amin Gholamzadeh1
Abstract: Biosurfactants are a group of surface active agents which are produced by microorganisms. In this research a bacterial specie s with the ability of producing biosurfactant was isolated from soil. The bacterium was able to grow on gas oil as the sole sou rce of carbon and energy. Concentrations of gas oil up to about two percent were not detrimental to the bacterial activity. The excess gas oil in the fermentation medium served as an exteractant for the biosurfactant. Separation of the organic phase from the a queous phase and evaporation of the gas oil resulted in a powder of crude biosurfactant. Each liter of the fermentation medium gave about 2.8 grams crude biosurfactant. The critical micelle concentration of the powder was 100 mg/L. The biosurfactant reduce d the surface tension of distilled water to 26mN/m. An aqueous solution of the biosurfactant was used to enhance oil recovery in a high permeable and a low per meable laboratory core. The solution enhanced the oil recovery up to 15% in the high permeable core at ambient temperature. This figure was
7.5% in the low permeable core. The biosurfactant retained its activity in enhanced oil recovery when the temperature of the core increased to 80 oC.
Key words: Biosurfactant, Critical Micelle Concentration, Extraction, Microbial Enhanced Oil Recovery.
1 INTRODUCTION
Biossurfactants are a group of surface active molecules that are produced by a variety of microorganisms. Like chemical sur- factants, biosurfactants have hydrophobic and hydrophilic moieties. The hydrophilic moiety of a biosurfactant is a carbo- hydrate (mono, di, or polysaccharides), an amino acid or a peptide, and the hydrophobic moiety is a saturated or unsatu- rated fatty acid and accordingly biosurfactants are classified as glycolipides, lipoproteins, lipopeptides, polymeric, and parti- culate biosurfactants [Don, et al, 2003. and Desai, et al, 1997] Due to environmental concerns about the use of chemical sur- factants, there is a growing interest to replace these chemicals by biosurfactants. The potential applications of biosurfactants have been examined in different areas. Biosurfactants have been used in flotation for the removal of metal ions, in biore- mediation, in enhanced oil recovery processes, in gene trans- fer, and in food, pharmaceutical, and cosmetic industries [Christova, et al. 2004, Strappa, 2004, Gregory, et al. 2004] Dif- ferent microbial species have been used in biosurfactant pro- duction. Among the species cited in the literature are Pseudo- monas putida [Strappa, et al, 2004, Ghadiri, 2003, Syyoun., et al,
2002], Actinomycetes, Bacillus subtilis, Lactococcus lactis, Lactoba- cillus strains [Rodrigues, et al, 2006, Rodrigues, et al, 2006.], Streptococcus thermophilus, Bacillus licheniformis , Pseudomonas
aeruginosa, Nocardioides sp. Bacillus pumilus, Aeromonas sp. Ser- ratia sp. Rhodococcus strains , and Candida ingens [Rodrigues, et al, 2006].
Both soluble and insoluble carbon substrates have been used
for biosurfactant production. Glucose, glycerol, cheese whey,
molasses are soluble substrates that have been tested for bio- surfactant production. Among insoluble substrates, different kinds of vegetable oils and different hydrocarbons have been used to produce biosurfactants. Insoluble substrates have been reported to be superior in biosurfactant production [Saikr-
shna, et al, 2007., Rodrigues, et al, 2006, Rodrigues, et al,
2006.].
The advantage of bacteria in oil recovery is a well known phe-
nomenon. Bacteria can enhance oil recovery in different ways.
They produce gases like carbon dioxide, organic solvents and
some surface active agents (biosurfactant). All these can help
oil mobilization in reservoirs. Active microorganisms can
grow in the reservoirs and block larger pores. This causes the fluid to pass through smaller pores in subsequent flooding. Three methods have been suggested for microbial enhanced oil recovery. In the first method biosurfactant and/or organic solvents are produced at the surface and the whole fermenta- tion medium is then introduced to the reservoir. In the second method proper microbes are introduced to the reservoir for in situ production of gases, solvents, and biosurfactants. In the third method only selected nutrients are injected to the reser- voir to stimulate the activity of indigenous bacteria [Rodri- gues, et al, 2006]. The disadvantage of injecting the whole fer- mentation medium to the wells is that the concentration of the useful components like organic solvents and biosurfactants might not be high enough to be effective. Moreover, the fer- mentation medium contains various constitutes like mineral salts and carbohydrates that contaminate the reservoir without contributing to enhanced oil recovery. The second and third methods are applicable only to those reservoirs having com- patible environmental conditions (pH, temperature, salinity, nutrient availability) for microbial growth. The drawback for all the three methods is that the efficiency of the methods is not predictable and field trial needs to be done for each specif- ic reservoir [Ghadiri, 2003, Syyoun, 2002, Gregory, 2002, Saikr- shna, 2007, Rodrigues, et al, 2006].
Ex situ production and purification of biosurfactants, and ap-
plication in enhanced oil recovery in known concentrations, is
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Biosurfactant Production by Microorganism for Enhanced Oil Recovery 2
ISSN 2229-5518
an alternative to the existing MEOR methods. Biosurfactants can replace the chemical surfactants. The application of bio- surfactants in purified or concentrated forms has not been widely investigated. It is generally believed that using biosur- factants in purified or concentrated forms are costly. In this research we propose a simple method for simultaneous pro- duction and separation of a biosurfactant and its application to enhanced oil recovery.
2 MATERIALS AND METHODS
2.1 Mineral medium
A mineral medium with the following composition was used for fermentation:
KH2PO4; 3.4 gr/lit, K2HPO4; 4.3 gr/lit , NaNO3; 4 gr/lit, MgCl2.7H2O; 0.2 gr/lit, CaCl2.2H2O; 0.04 gr/lit, FeSO4; 0.03 gr/lit, MnCl2; 0.001 gr/lit, NaMoO4; 0.002 gr/lit, CuSO4;
0.0001 gr/lit, H3BO3; 0.0003 g/lit, ZnSO4; 0.0015 gr/lit. All of the minerals were of analytical grade.
2.2 Carbon source
Gas oil was used as the sole source of carbon and energy for microorganisms. It was obtained from a gas station.
2.3 Isolation of biosurfactant producing microorgan-
isms
Soil samples were collected from different places in the cam- pus. Water was added to each sample and mixed thoroughly. The solid residues were filtered using a filter cloth. One milli- liter of each filtrate was added to a flask containing 50mL of

the mineral medium and 1mL gas oil. The flasks were incu- bated at 30oC. After 3 days the microbial growth was evident in some of the flasks. The flask with the highest turbidity was selected. The microbes from this flask were streaked on nu-
trient agar and stored at 4 C for later experiments.
2.4 Biosurfactant production

A 2L jar was used as the bioreactor. The jar had ports for air passage. For each experiment, the jar was filled with 1L min- eral medium and 4 mL gas oil. The jar was then inoculated with microorganisms. Air bubbled through the medium using a small air pump. The system was under ambient temperature
(25-30 C ). The fermentation lasted for four days and 4 mL gas oil was added to the medium in each day. After fermenta- tion, the whole medium was centrifuged. The microbial pellet was discarded. The organic phase was evaporated to obtain crude biosurfactant as a powder, and the aqueous phase was extracted once more with the half volume of gas oil to obtain any remaining biosurfactant.
2.5 Surface tension measurement
The surface tension of the liquid culture was measured using a digital tensiometer.
2.6 Core experiments
Two laboratory cores with the following characteristics were used to examine the effect of the biosurfactant in enhanced oil recovery.
Table 1: characteristics were used to examine the effect of the biosurfactant in enhanced oil recovery
Core No. | Length (cm) | Diameter (cm) | %Porosity | Permeability(mD) | %Saturated water |
1 | 38 | 6 | 20 | 56 | 25 |
2 | 38 | 4.5 | 40 | 2.5 | 18 |

Heavy oil (19 API ) was used for the experiment. Brine with
the following composition was used in flooding:
Table 2: Brine composition was used in flooding
Component | NaCl | KCl | MgCl2 | KBr | CaCl2 | NaHCO3 | Na2SO4 | NaF |
Concentration (gr /lit) | 22.53 | 0.7 | 4.2 | 0.031 | 1.16 | 0.221 | 4.1 | 0.003 |
The cores were washed with toluene for two days and dried in an oven. After that, the cores were under vacuum for four hours and were then saturated with water and a heavy crude oil successively. The cores were flooded with brine and then with the biosurfactant solution (2 gr/lit).
3 RESULTS AND DISCUSSIONS
3.1 Microorganism
Microscopic observation showed that the isolated microorgan- ism was a bacterium. Soil samples from garden are usually rich in bacteria which many of them are able to grow on hy- drocarbons as the sole source of carbon and energy provided that all necessary minerals are present. Preliminary tests


showed that the species was able to reduce the surface tension of the mineral medium from 51 mN.m 1 to 27 mN.m 1 indi- cating that it was a biosurfactant producer. The species were not identified
3.2 Biosurfactant production
Figure (1) shows the fermentation medium after it was centri- fuged. Two distinct phases can be seen. The remaining biosur- factant in aqueous phase was extracted with gas oil (Figure 2). The results of three replicate of the experiment have been pre- sented in table 3. Much of the biosurfactant accumulated in the organic phase. The organic phase could be separated from the aqueous phase using a decanter easily. Evaporation of the gas oil resulted in a white powder.
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Biosurfactant Production by Microorganism for Enhanced Oil Recovery 3
ISSN 2229-5518


Addition of excess gas oil was advantageous in extraction of the biosurfactant simultaneous to fermentation. Although ap- plication of concentrated biosurfactant has rarely been ex- amined in enhanced oil recovery, recovery of biosurfactants from fermentation media has been done for analytical purpos- es.
Figure (1): The fermentation medium after it was centrifuged
Figure (2): Extraction of the aqueous phase with gas oil Biosurfactants are usually separated from the fermentation medium by methods such as salting out (saturation of the fermentation medium for precipitation biosurfactant), solvent precipitation (addition of a miscible solvent to the fermenta- tion medium for precipitation of biosurfactant), and solvent extraction. All of the methods require addition of a material to the medium after fermentation. The method proposed here seems to be more economical than existing methods for bio- surfactant production.
3.2.1 The effect of concentration of minerals on biosur-
factant production
The composition of the mineral medium was adopted from reference ®. The medium was claimed to be excellent for hy- drocarbon degraders. Since the concentrations of some of the medium constituents are rather large, biosurfactant produc- tion was examined under lower concentrations.
Table3: Results of biosurfactant production using initial mineral medium in three runs
Run No. | Biosurfactant in organic phase (g) | Biosurfactant in aqueous phase after extraction with gas oil (g) | Total biosurfactant per liter of mineral medium (g) |
1 | 1.95 | 0.90 | 2.85 |
2 | 1.88 | 0.84 | 2.72 |
3 | 2.07 | 0.75 | 2.82 |
For this purpose two mineral media were prepared having all components but in concentrations half and one third of those mentioned above, respectively. Biosurfactant production in these media was compared with the initial medium. The re- sults are in Fig.3. Reducing the concentrations causes the bio- surfactant production to decrease. The decrease was not much significant when the concentrations of the minerals decreased to half.
3.3 Critical micelle concentration of the biosurfactant Different concentrations of the biosurfactant in water were prepared. The surface tensions of the solutions were measured and the critical micelle concentration of the biosurfactant was determined. Fig.4 shows the result. The critical micelle concen- tration of the biosurfactant is rather small (100 mg/L) express- ing its potential economical use in enhanced oil recovery.
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Biosurfactant Production by Microorganism for Enhanced Oil Recovery 4
ISSN 2229-5518

3 Intial medium
2.5
Medium w ith half concentration of the initial medium
2
1.5
1
0.5
Medium w ith one third concentration
of the initial medium
0
Figure (3): The effect of the minerals concentration on biosurfactant production
3.4 Using biosurfactant in enhanced oil recovery


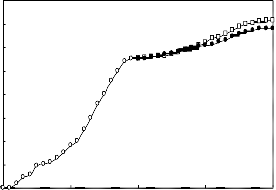
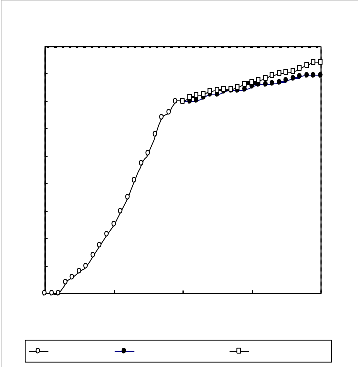
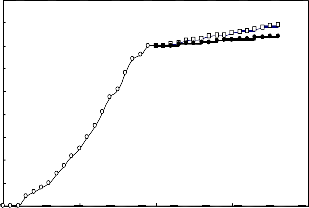
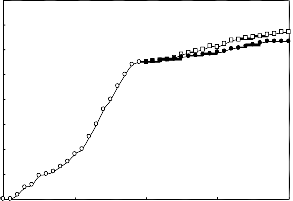
A 2g.L-1 solution of the biosurfactant in brine was prepared and used in enhanced oil recovery in laboratory cores. The recovery process was performed at temperatures 25 C and 80
C . The results are presented in Figures 5 to 8. In each case the effect of biosurfactant has been compared with a chemical surfactant (Triton x-100). The results indicate that the effect of the biosurfactant in oil recovery is comparable with that of Triton x-100.

80
70
60
50
CMC
40
30
20
80
70
60
50
40
30
20
10
0
0 500 1000 1500 2000

Time(min)
Water Flooding Surfactant Flooding Biosurfactant Flooding
10
0
1 10 100 1000 10000
Bios urfactant concentration (mg. L-1)
Figure (5): Oil recovery in the high permeable core at 25 oC
Figure (4): Critical micelle concentration of the biosurfactant
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Biosurfactant Production by Microorganism for Enhanced Oil Recovery 5
ISSN 2229-5518
18
18 16
14
16
12
14
10
12 8
10 6
8 4
2
6
0
4

2
0
0 500 1000 1500 2000
Time (min)
Water Flooding Biosurfactant Flooding Surfactant Flooding
0 500 1000 1500 2000
T ime (min)
Water Flooding Biosurfactant Flooding Surfactant Flooding
Figure (6): Oil recovery in the low permeable core at 25 oC
80
70
60
50
40
30
20
10
0
0 500 1000 1500 2000

Time (min)
Water Flooding Biosurf actant Flooding Surf actant Flooding
Figure (7): Oil recovery in the high permeable core at 80 oC
Figure (8): Oil recovery in the low permeable core at 80 oC
4 CONCLUSION
Gas oil is a proper substrate for biosurfactant production. It can serve as both substrate and extracting for the biosurfac- tant. The biosurfactant obtained here had the critical micelle concentration of 100 mg/L. The biosurfactant was successfully used in enhanced oil recovery in consolidated laboratory cores.
REFERENCE
[1] Christova, N., Tuleva, B., Lalchev, V., Jordnova, A., Jorda- nov, B., 2004. Rhamnolipid Biosurfactants Produced by Reni bacterium Salmoninarum 27BN During Growth on n- Hexadecane, Journal of Petroleum Science and Engineering
59c.pp70-74.
[2] Desai, J., Banat I., 1997. Microbial production of surfactants
and their commercial potential. Microbial Mol Biol Rev. 47-64. [3] Don, G., Paul, W., Willhite, G., 2003. Enhanced Oil Recov- ery, Chapter 5, and Society of Petroleum Engineers Textbook Series Vol.6: P 26-186.

[4] Ghadiri, N. A , 2003. Biosurfactant production In MEOR for

Improvement of Iran, Oil Reservoirs SPE84907.
[5] Gregory, A. Bala, Debby F. Bruhn, Sandra L. Fox, Karl
S.Noha, and David N., 2002. Microbiogical Production of
Surfactant From Agricultural Resiualls for IOR, SPE75239.
[6] Rodrigues, L., Teixeira, J., Oliveira, R., Vandermei, H.C.,
2006. Response surface optimization of the medium compo-
nents for the production of biosurfactants by probiotic bacte-
ria, process Biochemisity 41, 1-10.
[7] Rodrigues, L., Moldes, A., Teixeira, J., and Oliveira, R.,
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Biosurfactant Production by Microorganism for Enhanced Oil Recovery 6
ISSN 2229-5518
2006. Kinetic study of fermentative biosurfactant production by Lactobacillus strains, Biochemical Engineering journal 28,
109-116.
[8] Saikrshna, M., Knapp, R. M., Mclnerney, M. J., 2007. Mi-
crobial Enhanced Oil Recovery Technologies, A Review of the
Past, Present,and Future, SPE 106978.31.
[9] Strappa, L.A., De Lucia, J.P., Maure, M.A., and Lipoiz, M.L.L., 2004. A Novel and Successful MEOR Project in Strong Water - Drive Reservoir Vizcacheras Field, Argentina, SPE89456.
[10] Syyoun, M. H., 2002. Microbial Enhanced Oil Recovery,
Research studies in the Arabic area during the Last 10 years. SPE 75218.
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
IJSER © 2012
http://www.ijser.org







