International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 654
ISSN 2229-5518
Biodiesel Fuel from Differently Sourced Local Seed Oils: Characterization, Effects of Catalysts, Total Glycerol Content and Flow
Rates
Anuoluwa A. Akinsiku*, Enock O. Dare, Michael S. Ayodele, Fatai O. Oladoyinbo, Kehinde A. Akinlabi, Kolawole O. Ajanaku, Tolutope O. Siyanbola and Joseph A. Adekoya.
Abstract— The recently observed depletion in the petroleum resources, which also mainly constituted carbon dioxide emission and global warming problems call for renewable and sustainable alternative fuels. Oils were extracted from various seeds: Jatropha curcas (Botuje),
Pentaclethra macrophylla (Apara) and soybean, using petroleum ether (40-60℃). Alkali catalyzed transesterification of the oils (biodiesel pro-
duction) in the presence of different kinds of alcohol (methanol, ethanol and propanol) were carried out using sodium hydroxide as catalyst.
In the case of Jatropha oil, potassium hydroxide served as catalyst. Effect of catalysts to obtain optimum biofuel was established. In the case
of soybean oil, fatty acid methyl ester, FAME, (96%), fatty acid ethyl ester, FAEE, (84%) and fatty acid propyl ester, FAPE, (37.50%) were pro- duced. In waste palm kernel oil, methyl ester (72.92%) and ethyl ester (46.25%) were obtained. In refined palm kernel oil, methyl ester (70.83%), ethyl ester (66.67%) and (14.17%) propyl ester were produced. However, only methyl ester conversion (20.83%) was possible in Pen- taclethra macrophylla oil. In Jatropha curcas using KOH catalyst, only methyl ester (80%) formation was possible. Moreover, yields were af- fected as the alcohol alkyl became bulkier giving relatively lower value of biodiesel. Sulphur content (0.01) obtained for each of the biofuel was satisfactory when compared with ASTM standard (0.05 maximum). The cetane value of soybean oil (45.5), refined palm kernel oil (46) and used oil (44.6) were quite reasonable compared with the special standard (47). The combustion energy of the fuels from refined palm kernel oil, waste palm kernel oil and soybean are 39, 36 and 45.5 respectively. The total glycerol content (Gc) of the methyl and ethyl esters emanat- ed from soybean are quite reasonable and fell within standard.
Keywords: biodiesel, flow rates, local seed oils, total glycerol content, transesterification
1 INTRODUCTION
—————————— ——————————
Among liquid biofuels, biodiesel is gaining acceptance and mar- ket share as diesel fuel in Europe and the United states. Biodiesel has become more attractive recently because of its environmental benefits and the fact that is made from renewable resources [1]. Moreover, they have practically no sulfur content, offer no storage difficulty, and they have good lubrication properties.
The substitutions of petrodiesel oil by renewable fuels produced within some countries generate high foreign exchange savings, even for the major oil exporting countries. Consequently, project or research in this direction potentially would serve to solve eco- nomical problems and improve economy of developing countries.
Considerable research has been done on vegetable oils as diesel fuel. Feedstock commonly used includes: palm oil, sunflower oil,
——————————————
Corresponding Author: Akinsiku, Anuoluwa Abimbola Chemistry Department, Covenant University, Canaanland, Ota. P.M.B.1023, Ota, Ogun State, Nigeria.
E mail: anu.akins iku@covenant uni versity.edu.ng omosotomi2000@yahoo.com
coconut oil, rapeseed oil and tung oil. Animal fats, although men- tioned frequently, have not been studied to the same extent as vegetable oils. Some methods applicable to vegetable oils are not applicable to animal fats because of natural property differences. Oils from algae, bacteria and fungi also have been investigated [2]. Microalgae have been examined as a source of methyl ester diesel fuel [3]. Terpenes and latexes also were studied as diesel fuels [4].
Some natural glycerides contain higher levels of unsaturated fatty acids. Their direct uses as biodiesel fuel are precluded by high viscosities. Fats, however, contain more saturated fatty acids. They cannot be used as fuel in a diesel engine in their original form. Because of the problems, such as carbon deposits in the engine, engine durability and lubricating oil contamination, asso- ciated with the use of oils and fats as diesel fuels, they must be derivatized to be compatible with existing engines. Therefore, the work reported herein describe the formation of biodiesel in its various derivatives (methyl-, ethyl- and propyl-) from local seed oils while studying the effects of catalysts and glycerol as it affect flow rates on conversion.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 655
ISSN 2229-5518
2 MATERIALS AND METHODS
2.1 Seeds collection, extraction and analysis
Seeds were collected from different locations. Pentaclethra micro- carpa (Apara) seeds were collected from Ijebu ode and Lafenwa market in Abeokuta, Ogun state. Refined palm kernel oil (PKO) was bought from Adatan market, Abeokuta. Soybean was ob- tained from Kuto market in Abeokuta, Ogun state. Waste/used cooking oils were obtained from different beans cake sellers from Sabo and Carwash; Abeokuta, in Ogun state. Jatropha curcas was collected from University of Agriculture Abeokuta and Asero, Abeokuta in Ogun state. These places are located in the Western part of Nigeria.
The seed shells were cracked to remove the seeds, sun dried and later oven dried to constant weight. Oil from the seeds was ob- tained by the method of soxhlet extraction using petroleum ether (40-60OC).
Physical analyses of vegetable oils were conducted according to the AOCS standard test methods. The following parameters: io- dine value, acid value, peroxide value, saponification value, re- fractive index and free fatty acid were determined on each sample of the extracted oil as well as used/ waste cooking oil. These rou- tine analyses on different seed oils were carried out using AOCS (1978) and Cordex Alimentarious (CAC/RM 9/14-1969).
2.2 Alkali- catalyzed transesterification process
2.2.1 Preparation of Alkoxide
The solvents (methanol, ethanol and propanol) were distilled be- fore use to ascertain purity. Sodium methoxide, ethoxide and propoxide (Na+CH3O-) were prepared by dissolving 0.20g, NaOH (catalyst) in 20ml methanol, 20ml ethanol or 20ml propanol re- spectively. The solutions were thoroughly mixed until the whole alkali dissolved in each solvent. The procedure was repeated us- ing 0.35g, 0.50g, 0.75g and 1.0g NaOH.
Also, potassium methoxide, ethoxide and propoxide (K+CH3O-)
were created by mixing 0.20g KOH (catalyst) in 20ml methanol,
0.20g KOH in ethanol and 0.2g KOH in 20ml propanol respective- ly. The procedure was repeated by dissolving 0.35g, 0.50g, 0.75g and 1.0g KOH in methanol, ethanol or propanol.
2.3 Fuel Characterization
The fatty acid alkyl esters were characterized for their physico- chemical and fuel properties. The parameters includes: total acid value, cetane number (CN), iodine number and saponification value. Chemical analyses of vegetable oils were conducted accord- ing to the standard test methods: Bomb calorimeter was used to determine HHV in KJ/g, ASTM D613 for CN and ASTM D5453 for sulphur content, while AOCS (1978) and Cordex Alimentarious (CAC/RM 9/14-1969) was used for IV, saponification value (SV), acid value and free fatty acid. Instrumental analyses of the bio-
diesel fuel were carried out by Nigeria liquefied Natural Gas company (NLNG), Bonny Island at International Energy Services Limited. Port Harcourt, in Nigeria.
Total glycerol content Gs (wt% on mass of biodiesel fuel) was de-
termined using the formula:
Gs = 0.1044WTG + 0.1488WDG + 0.2591WMG + WG, where WTG, WDG,
WMG and WG are amounts of triglycerides, diglycerides, mono- glycerides and free glycerol respectively.
3 RESULTS AND DISCUSSION
Biodiesel has become more attractive recently because of its envi- ronmental benefits and the fact that it is made from renewable resources [1].
In this study, potential of locally sourced seed oils and waste/used cooking oils were established for the development of biodiesel using alkali-catalysed transesterification. The transester- ification experiments using methanol, ethanol and propanol (sol- vents) were carried out on oils from; Jatropha curcas (Botuje), Pen- taclethra macrophylla (Apara) and soybean. Refined palm kernel oil and used palm kernel oil were used as well. Sodium hydroxide (NaOH) was used as catalyst in all the oils for transesterification processes with the exception Jatropha oil where potassium hy- droxide (KOH) was used instead. The two catalysts were used effectively with the hope to optimizing biodiesel conversion con- dition. The catalysts exhibited a pronounced effect on methyl es- ter, ethyl ester and propyl ester formation with evidence of good conversion. However, in the transesterification process, addition of excess amount of catalyst gave rise to the formation of an emul- sion, which increased the flow rate and led to the formation of gels. Ramadhas, et al., reported [5] failure in transesterification process and the phenomenon was attributed to insufficient amount of catalyst added.
Alkali-catalysed transesterification of soybean oil was studied. Successful transesterification reaction produced two liquid phas- es: ester and glycerine. Phase separation was observed within 5 minutes. Complete separation took about 20 hours as expected in the alkali-catalyzed transesterification process.
3.1 Effect of catalyst (NaOH) on Ester Formation
Using soybean oil
The transesterfication of soybean oil with methanol, ethanol and butanol, using 1% concentrated sulfuric acid, was unsatisfactory when the molar ratios were 6:1 and 20:1 [6]. A 30:1 ratio resulted in a high conversion to methyl ester. In this study, alkaline trans- esterification of soybean oil using NaOH in methanol, ethanol and propanol was considered to produce sodium alkoxide (Na+ CH3O-
).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 656
ISSN 2229-5518
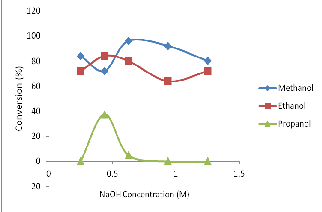
Fig. 1: Effect of NaOH (catalyst) on biodiesel conversion from soybean oil in different solvent media.
Result in figure 1 indicates highest conversion (96%) in the case of methyl ester derivative of the oil at catalyst concentration of 6.25 x
10-1M. Lower conversions were also obtained at catalyst concen-
trations of 4.38 x 10-1M and 0.25 x 10-1 M giving 72% and 84% for The seed shells were cracked to remove the seeds, sun dried and later oven dried to constant weight. Oil from the seeds was ob- tained by the method of soxhlet extraction using petroleum ether (40-60OC).
Physical analyses of vegetable oils were conducted according to the AOCS standard test methods. The following parameters: io- dine value, acid value, peroxide value, saponification value, re- fractive index and free fatty acid were determined on each sample of the extracted oil as well as used/ waste cooking oil. These rou- tine analyses on different seed oils were carried out using AOCS (1978) and Cordex Alimentarious (CAC/RM 9/14-1969).
2.2 Alkali- catalyzed transesterification process
2.2.1 Preparation of Alkoxide
The solvents (methanol, ethanol and propanol) were distilled be- fore use to ascertain purity. Sodium methoxide, ethoxide and propoxide (Na+CH3O-) were prepared by dissolving 0.20g, NaOH (catalyst) in 20ml methanol, 20ml ethanol or 20ml propanol re- spectively. The solutions were thoroughly mixed until the whole alkali dissolved in each solvent. The procedure was repeated us- ing 0.35g, 0.50g, 0.75g and 1.0g NaOH.
Also, potassium methoxide, ethoxide and propoxide (K+CH3O-)
were created by mixing 0.20g KOH (catalyst) in 20ml methanol,
0.20g KOH in ethanol and 0.2g KOH in 20ml propanol respective- ly. The procedure was repeated by dissolving 0.35g, 0.50g, 0.75g and 1.0g KOH in methanol, ethanol or propanol.
2.3 Fuel Characterization
The fatty acid alkyl esters were characterized for their physico- chemical and fuel properties. The parameters includes: total acid
value, cetane number (CN), iodine number and saponification value. Chemical analyses of vegetable oils were conducted accord- ing to the standard test methods: Bomb calorimeter was used to determine HHV in KJ/g, ASTM D613 for CN and ASTM D5453 for sulphur content, while AOCS (1978) and Cordex Alimentarious (CAC/RM 9/14-1969) was used for IV, saponification value (SV), acid value and free fatty acid. Instrumental analyses of the bio- diesel fuel were carried out by Nigeria liquefied Natural Gas company (NLNG), Bonny Island at International Energy Services Limited. Port Harcourt, in Nigeria.
Total glycerol content Gs (wt% on mass of biodiesel fuel) was de-
termined using the formula:
Gs = 0.1044WTG + 0.1488WDG + 0.2591WMG + WG, where WTG, WDG, WMG and WG are amounts of triglycerides, diglycerides, mono- glycerides and free glycerol respectively.
4 RESULTS AND DISCUSSION
Biodiesel has become more attractive recently because of its envi- ronmental benefits and the fact that it is made from renewable resources [1].
In this study, potential of locally sourced seed oils and
waste/used cooking oils were established for the development of biodiesel using alkali-catalysed transesterification. The transester- ification experiments using methanol, ethanol and propanol (sol- vents) were carried out on oils from; Jatropha curcas (Botuje), Pen- taclethra macrophylla (Apara) and soybean. Refined palm kernel oil and used palm kernel oil were used as well. Sodium hydroxide (NaOH) was used as catalyst in all the oils for transesterification processes with the exception Jatropha oil where potassium hy- droxide (KOH) was used instead. The two catalysts were used effectively with the hope to optimizing biodiesel conversion con- dition. The catalysts exhibited a pronounced effect on methyl es- ter, ethyl ester and propyl ester formation with evidence of good conversion. However, in the transesterification process, addition of excess amount of catalyst gave rise to the formation of an emul- sion, which increased the flow rate and led to the formation of gels. Ramadhas, et al., reported [5] failure in transesterification process and the phenomenon was attributed to insufficient amount of catalyst added.
Alkali-catalysed transesterification of soybean oil was studied.
Successful transesterification reaction produced two liquid phas- es: ester and glycerine. Phase separation was observed within 5 minutes. Complete separation took about 20 hours as expected in the alkali-catalyzed transesterification process.
4.1 Effect of catalyst (NaOH) on Ester Formation
Using soybean oil
The transesterfication of soybean oil with methanol, ethanol and butanol, using 1% concentrated sulfuric acid, was unsatisfactory when the molar ratios were 6:1 and 20:1 [6]. A 30:1 ratio resulted in a high conversion to methyl ester. In this study, alkaline trans-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 657
ISSN 2229-5518
esterification of soybean oil using NaOH in methanol, ethanol and propanol was considered to produce sodium alkoxide (Na+ CH3O-
).
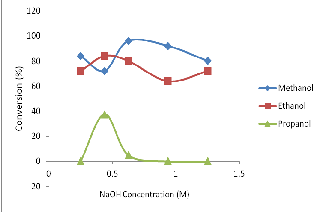
Fig. 1: Effect of NaOH (catalyst) on biodiesel conversion from soybean oil in different solvent media.
Result in figure 1 indicates highest conversion (96%) in the case of methyl ester derivative of the oil at catalyst concentration of 6.25 x
10-1M. Lower conversions were also obtained at catalyst concen-
trations of 4.38 x 10-1M and 0.25 x 10-1 M giving 72% and 84% for methyl ester derivatives respectively.
Moreover, the experiment optimally produced ethyl ester at 4.38 x
10-1M NaOH concentration, with 84% conversion. Reasonable biodiesel yields are also obtained at the catalyst concentrations of
0.25 x 10-1M, 6.25 x 10 -1M, 9.38 x 10 -1M and 12.5 x 10-1M NaOH
concentrations with ethyl ester conversions of 72%, 80%, 64% and
72% respectively.
However, propyl ester was obtainable at catalyst concentrations of
4.38 x 10-1M and 6.25 x 10-1 M only. With 4.38 x 10-1M catalyst con-
centration, 37.50% conversion resulted which was its highest. This result of propyl ester formation was relatively low.
Ayhan in 2002 reported that catalyst improved the yield and rate of transesterification. This is observed in methyl ester, ethyl ester and propyl ester production using soybean oil. The reduction in the trend of ester yield from methanol to propanol media plausi- bly due to stearic effect on the hydrocarbon chain and polarity of the alcohol.
4.2 Effect of catalyst (NaOH) on transesterification of used/waste palm kernel oil (PKO)
Figure 2 reveals the significant effect of catalyst on biodiesel for- mation from used or waste PKO. The alcoholysis of used/waste cooking oil was successful in methyl ester and ethyl ester for- mation but failed in propanol as solvent. At NaOH concentration of 0.25 x 10-1 M, methyl ester produced was insignificant, and this shows that the catalyst concentration was not enough to break the
fatty acid in the triglyceride of waste oil [5].
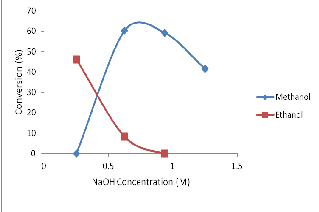
Fig. 2: Effect of NaOH (catalyst) on transesterification of waste oil in different solvents.
However, at increased concentrations of 4.38 x 10-1M, 6.25 x 10-1M,
9.38 x 10 -1M and 12.5 x 10-1M, conversions of 72.92%, 60.40%,
59.25% and 41.67% respectively were obtained. Optimal condition for methyl ester production in waste oil was evident at 4.38 x 10-
1M NaOH (catalyst) concentration, with 72.92% conversion.
Ethyl ester formation in waste oil was highest at a very low cata-
lyst concentration of 0.25x 10-1M with 46.25% yield.
However, propyl ester formation was not successful. Thus, only methyl ester and ethyl esters were obtained from used cooking oil using alkali-catalyzed transesterification method.
4.3 Effect of catalyst on biodiesel formation in refined palm kernel oil using NaOH (catalyst) in alcohol
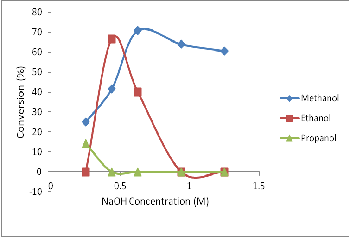
Figure 3 is the graph showing optimal condition for methyl ester, ethyl ester and propyl ester conversion as a function of catalyst in refined palm kernel oil. The amount of catalyst used in transester- ification process affects the efficiency of the process as reported by Ramadhas, et. al., [5].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 658
ISSN 2229-5518
Fig. 3: Effect of NaOH (catalyst) on biodiesel conversion from refined palm kernel oil in different solvent media.
Methyl ester was produced at NaOH concentrations of 0.25 x 10-
1M , 4.38 x 10-1M , 6.25 x 10-1M, 9.38 x 10 -1M and 12.5 x 10-1M, with biodiesel conversions of 25%, 41.67%, 70.83%, 64% and 60.42% respectively.
Moreover, ethyl esters were obtained at two NaOH (catalyst) con- centrations; 4.38 x 10-1M and 6.25 x 10-1M, with conversions of
66.67% and 40% respectively.
Propyl ester was obtained at 0.25 x 10-1M and 4.38 x 10-1M catalyst
concentrations. The percentage conversions at these concentra- tions were 10% and 14.17% respectively. However, these conver- sions were very low relatively.
Thus, optimal biodiesel conversion was evident at NaOH concen- tration of 6.25 x 10-1M (70.83%) in CH3OH, 4.38 x 10-1M (66.67%) in C2H5OH and 4.38 x 10-1M (14.17%) in C3H7OH.
4.4 Effect of catalyst on biodiesel formation in
Pentaclethra macrophylla (Apara)
Pentaclethra macrophylla (Apara); locally sourced seed oil was in- vestigated as an alternative feedstock for fatty acid alkyl ester production. The oil content of 45% proves the oil to be promising as one of the feedstock considered for biofuel. This is because seeds with high oil content are considered for biodiesel as report- ed by Karaosmanoglu [7].
Biodiesel production using Pentaclethra macrophylla was not satis- factory. Only methyl ester conversion was possible at 0.25 x 10-1M NaOH concentration with 20.83% conversion. At other catalyst concentrations, in different solvent media; ethanol and propanol, there was no evident of biodiesel conversion.
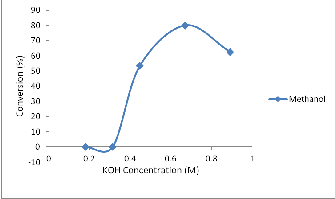
Fig 4: Effect of KOH (catalyst) on biodiesel formation from
Jatropha oil
The effect of catalyst (KOH) in methanol, ethanol and propanol media is shown in figure 4. Only methyl ester formation was pos- sible in Jatropha curcas using KOH catalyst. NaOH Catalyst was unable to catalyze the reaction. Optimal yield was obtained at 6.70 x 10-1M concentration of the catalyst with 80% conversion. At oth-
er catalyst concentrations of 4.46 x 10-1M and 8.90 x10-1M; conver- sions of 53.33% and 62.50% were obtained respectively. There was no evidence of fuel formation in ethanol and propanol solvent media.
4.5 Biodiesel fuel properties
Table 1: Characteristics of biodiesel fuel from differently sourced feedstock.

ND: Not determined
The results of the analyses shown in table 1 reveals sulphur con- tent of 0.01 for all the biodiesel emanated from soybean oil, Penta- clethra oil, Jatropha oil, used palm kernel oil and refined palm kernel oil. The maximum sulphur content according to USA ASTM D-6751 is 0.05. Judging from this view, the fuels are satis- factory due to low sulphur in them. This means that they are not prone to knocking in combustion ignition engine [8].
Cetane number is a measure of the ignition performance of a die- sel fuel obtained by comparing it to reference fuels in standard- ized engine test. The higher the cetane number, the better the igni- tion ability of the fuel. Cetane number scale of diesel fuel covers the range from 0- 100. However, typical testing is in the range of
30-65 cetane number. (Destination: D 613-05). The cetane number of biodiesel varies, usually in the range of 48-67 depending on the feedstock [9]
The cetane number of biodiesel fuel from soybean oil, waste/used
cooking oil and palm kernel oil were found to be 45.5, 44.6 and 46 respectively. Therefore, the cetane value of soybean oil and that of palm kernel oil are quite reasonable compared with the special standard (47). Niehaus et. al. [10] and Schwab et. al., [11] reported the cetane number of biodiesel from soybean oil that was thermal- ly decomposed and distilled in air and nitrogen sparged with a standard ASTM distillation apparatus to be 43.3. Fangrui et. al. reported [12] that the cetane number of transesterification reaction is still better and higher than those of thermally cracked oil. This
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 659
ISSN 2229-5518
is demonstrated in this study where the value of cetane number of soybean oil is 45.5, higher than biodiesel obtained by thermal de- composition.
However, from table 1, the combustion energy (high heating en-
ergy) of the fuels from refined palm kernel oil, waste palm kernel oil and soybean are; 39, 36 and 45.5 respectively. Biodiesel ob- tained from soybean oil has high combustion power of 45.5.
4.6 Biodiesel flow rates
The essence of transesterification process is to lower the viscosity of oil. Table 2 shows the flow rates of feedstock with their corre- sponding biodiesel at their optimal conditions. Flow rate is de- pendent on viscosity. It is evident that there is reduction in the viscosity of biodiesel compared with their corresponding feed- stock, due to the increase in the flow rate of biodiesel compared with their corresponding feedstock. These results further indicate a complete transesterification and derivatization.
TABLE 2: Comparison of the Flowrates of Biodiesel
Fuel with Feedstock Input

Flow rate (cm3/min)
ing feedstock is encouraging and may not constitute blockage in diesel engine.
4.7 Total Glycerol content (Gc) of soybean biodiesel
Among the specification standards of biodiesel fuel, total glycerol content(Gc) is one of the most important characters, since glycer- ides significantly affect other fuel properties such as viscosity, pour point, amount of carbon residue etc. causing problems on filterability and deposition on the injection and combustion chamber.
4 CONCLUSIONS
Of the several methods available for producing biodiesel, trans-
esterification of natural oils and fats is currently the method of choice. The purpose of the process is to lower the viscosity of the oil or fat. Although blending of oils and other solvents lowers the viscosity, engine performance problems, such as carbon deposit and lubricating oil contamination, still exist. In this present study, a high efficient transesterification has been described; optimal condition for alkyl conversion in each sample oil has been deter- mined. Therefore, catalysts concentration significantly affects overall conversion of biodiesel. It has been evaluated in this work also the influence of various fatty acid derivatives (methyl-, ethyl-
Feedstock Original oil Methyl
Ethyl
Propyl and propyl esters) on biodiesel flow rate. Methyl esters of most of the oils increases oil flow rates significantly when compared to
ester
ester
ester
other.

The results on the total glycerin content of the biodiesel emanated
Jatropha curcas 2.00 3.67 - -
from soybean are quite impressive and conform favourably to standard. This phenomenon hopefully is another factor that would justify preclusion of unwanted combustion chamber depo-
Pentaclethra macro-
phylla
1.60 2.50 - -
sition and filterability problems.
5 AKNOWLEGMENTS
Waste palm kernel oil 2.40 4.00 2.00 1.00 palm kernel oil 2.33 2.33 2.00 1.00
Soybean oil 2.30 3.63 2.50 0.50
The authors want to use this medium to appreciate;
• The Research Development Centre (RESDEC), Universi- ty of Agriculture, Abeokuta; for their financial support.
• International Energy Services Limited, Port Harcourt Ni- geria.
• Nigeria Liquified Natural Gas (NLNG), Rivers State, Ni- geria.
Result obtained (table 2), to a large extent shows significant in-
crease in the flow rate of the biodiesel as the oil was transformed.
The result in the case of methyl ester from Jatropha (3.67), methyl ester from used oil (4.00), ester from refined palm kernel oil (3.33), ester from soybean oil (3.63) and ester from Pentaclethra oil (2.50), clearly indicates transesterified oil in its various derivatives. In all cases, FAME exhibited a general increase in flow rate over other derivatives (FAEE, FAPE) and this phenomenon accounts for re- duction in oil viscosity.
The increase in the flow of ester compared with their correspond-
6 REFERENCES
[1] E. Saucedo, “Biodisel”. Ingeniera Quimica 20, 19-29, (2001).
[2] E.G. Shay, “Diesel fuel from vegetable oils: status and oppor-
tunities” Biomass and Bioenergy.4:227-242,(1993).
[3] N. Nagel and P. Lemke, “Production of methyl fuel from
miceoalgea”. Appl.Biochem. Biotechnol. 24. 355-361. (1990).
[4] M. Calvin, “Fuel oils from higher plants.” Ann. Proc. Phyto-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 660
ISSN 2229-5518
chem. Soc. Eur. 26: 147-160, (1985).
[6] A.S. Ramadhas, C. Jayaraj, C. and Murale edharan, “Bio-
diesel Fuel from High FFA seed oil.” Fuel. 84: 335-340, (2005).
[7] B. Freedman, E.H. Pryde, and T.L. Mounts, “Variables af-
fecting the yields of highly unsaturated triglycerides”. JAOCS. 40:

197-201, (1984).
[8] F. Karaosmonoglu, “Vegetable oil fuels”: a review. Energy
Sources. 21: 221-31, (1999).
[9] Destination D. D6584-00; 1-17. Test method for determination
of free and total glycerine in B-100 biodiesel methyl esters by gas chromatography.
[10] S. Abdurrahman, D. Zahir, K. Canan, B.K. Aylin and H. Candan, “Transesterified Oil as a Biodiesel Fuel”, Bioresource Tech- nology: 99; 6656-6660, (2007).
[11] R.A. Niehaus, C.E. Goering, L.D. Savage, and S.C. Sorenson, Cracked Soybean oil as a fuel for a diesel engine. Trans. ASAE . 29:
683-689, (1986).
[12] A.W. Schwab, M.O Bagby and B. Freedman, “Preparation and properties of Diesel fuel from vegetable oils”. Fuel.66: 1372-
1378, (1987).
[13] M.A. Fangrui, A. Milford and Hanna, “Biodiesel production:
a Review. Fatty esters from transesterified vegetable oils”. JAOCS.
61: 1638-1643, (1999).
[14] ASTM, Pennsylvania. American Society for Testing and Ma-
terials (ASTM), 2000.
[15] D. Ayhan, “Biodiesel Fuel from Vegetable Oils Via Catalytic
and Non- Catalytic Supercritical Alcohol Transesterifications and
Other Methods: a survey.” Energy Conversion and Management 44:
2093-2109, (2002).
[16]. L. Donald, Pavia, M. Gary, Lampam, S. George, and Kriz, Jr. Introduction to Spectroscopy. Department of Chemistry, Western Washington University, Washington, US. 23-33, (1979).
[17] F. Ma, L.D. Clements, and M.A. Hannah, The effects of cata-
lyst, free fatty acids and water on transesterification of beef tallow. Trans, ASAE 41, 1261-1264, (1998).
IJSER © 2013 http://www.ijser.org




