International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 95
ISSN 2229-5518
Sarwat Jahan*, Shamim Akhtar***, Arfa Kamil*, Aneela Karim*, Mehwish Wajdi*, Zafar Saied Sai- fy**, Darkhshan Jabeen Haleem**, Nausheen Alam*
*Department of Pharmaceutical Chemistry, Faculty of
Pharmacy, Federal Urdu University of Arts, Sciences
and Technology, Gulshan-e- Iqbal, Karachi-75300, Pakistan, *Department of Pharmacology, University of Karachi-75270, Pakistan
**HEJ Research Institute of Chemistry, University of
Karachi-75270, Pakistan
*** Department of Pharmaceutical Chemistry, Facul-
ty of Pharmacy, University of Karachi- 75270, Paki- stan
Derivatives of alkyl piperidine were synthesized and were evaluated for potential anti-depressant and anti- psychotic activities in albino mice. The derivatives Ia- If and IIa-IIf, containing nitro, fluoro, chloro, bromo
of behavioral activity of small animals for the deter- mination of behavior [10].
Substituted alkyl piperidine has been a rich source of
numerous pharmacologically active drug substances since several decades [11-15]. Due to their often bio- logical activities, optically active piperidine alkaloids containing a stereogenic carbon atom at the 2-position are an important group of natural products and they have been the target of a number of synthetic strate- gies [16-20].
It was also reported that series of N-phenyl piperidine analogs were active and very potent versus wild-type HIV-1 and a broad range of NNRTI-resistant mutant viruses [21].
Therefore a huge amount of efforts have been devoted to their construction by synthetic chemists all over the world [22-34].
Different patterns of behavioral disorders include anx-
IJSER
and methoxy groups possessed significant activity in open field test when tested at the dose of 50 mg/kg body weight. It is also evident that the number of nitro groups, their positions in the phenyl ring and the other functional groups has relationships among them to impart certain activity to the molecules to which they are attached. The structures of the synthesized com- pounds were confirmed through different spectral techniques EI-MS, 1HNMR, IR and UV.
Any change in the levels of neurotransmitters influ- ence the behavior like locomotion, attitude, gripping, exploration etc. Various approaches such as latency to move and number of square crossed are the methods of choice to study locomotion and exploratory behav- ior [1-8] .
Open field activity test method is a precise established method to investigate the behavioral changes in mice
[9]. This method has been used for the measurement
iety, confusion, depression, agitation and insomnia
which are due to the deficiency or increase in biogenic amines or impair neurotransmission. Any change in the levels of neurotransmitters influence the behavior like locomotion, attitude, gripping, exploration etc. Various approaches such as latency to move and num- ber of square crossed are the methods of choice to study locomotion and exploratory behavior. EXPERIMENTAL
White Albino mice of either sex (locally bred) weigh- ing between 20-30 gm, purchased from Agha Khan Medical University and Hospital, Karachi were em- ployed for behavioral activity. All solvents such as DMSO and ethyl alcohol were of analytical grade. Disposable insulin syringes were used for intra perito- neal route.
Behavioral studies were performed in an open field apparatus mentioned below in the Research Institute of Pharmaceutical Sciences, Faculty of Pharmacy, and University of Karachi, Pakistan.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 96
ISSN 2229-5518
The open field apparatus composed of a square area
76×76 cm with walls 42 cm high. Floor of the appa- ratus was divided by lines into 25 equal squares. The mice were exposed to the open field after 30 minutes of receiving injection. The activity was scored as number of squares crossings with all four paws for 5 minutes [35]
Different patterns of behavioral disorders include anx- iety, confusion, depression, agitation and insomnia,
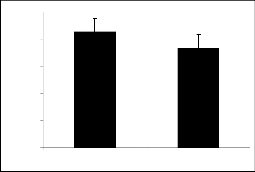
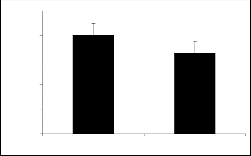
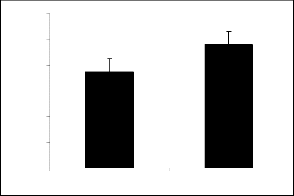
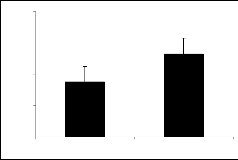
Derivatives of parent I showed variable responses (figure 1a and figure 1b). Compounds Ia and Ib ex- hibited the similar response. It means introduction of nitro and bromo groups at meta position in the phenyl ring attenuated the activity initially present in the par- ent compound.
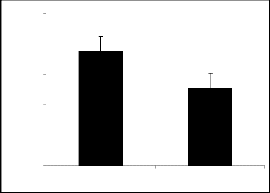
Compounds Ic (figure 1c), Id (figure 1d), Ie (figure
The results obtained among the derivatives of piperi- dine-2-methanol, interestingly it is evident that the at- tachment of nitro, bromo, flouro and chloro groups at position 4 (para position) in the phenyl ring made the derivatives successful to produce exploration and lo-
comotory behavior significantly. They have potentiat-
IJSER
which are due to the deficiency or increase in biogenic
amines or impair neurotransmission [36] [37].
Open field activity test method is a precise established method to investigate the behavioral changes in mice [9]. This method has been used for the measurement of behavioral activity of small animals for the deter- mination of behavior [38] [31][32] [39] and[13] [40] and [41].
Mixed strains of albino mice administering six deriva- tives (Ia, Ib, Ic, Id, Ie and If) of compound I and six derivatives (IIa, IIb, IIc, IId, IIe and IIf) of com- pound II when exposed to open field test for 5 minutes after 30 minutes of administration through intra peri- toneal route showed variable results.
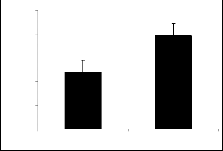
The results of parent molecules (compound I and compound II) are shown in tables 1, and 2, respective- ly while tables 1a to 1f and tables 2a to 2f presented the results of their derivatives (50 mg/kg) with corre- sponding figures.
Piperidine-2-methanol (I) and piperidine-2-ethanol (II) were tested at the dose of 50mg/kg body weight showed significant exploratory behavior and locomo- tion in the open field test (figure 1 and figure 2).
ed the effects of their parent compounds.
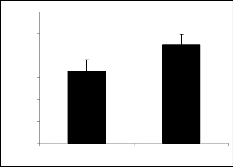
Compounds IIa (figure 2a), IIc (figure 2c) and IId (figure 2d) were less effective to change the explora- tory and locomotion activity as compared to control. Compounds IIb (figure 2b), IIe (figure 2e) and IIf (figure 2f) showed very significant increase in the ac- tivity of locomotion and exploration as far as the con- trol is concerned.
Comparing the derivatives of parent I, its para nitro derivative exhibited pronounced activity whereas, re- sults of the derivatives of parent II having nitro groups at different positions revealed variable results. The derivative having nitro group at meta and para posi- tions (IIc and IId respectively), there is less signifi- cant activity while the derivative having nitro group at ortho position (IIe) attained highly significant activity. The results revealed that position of nitro group in the phenyl ring may have definite effect on the activity. Compound having two nitro groups at meta positions (IIf) was responsible to cause more pronounced change in the behavior.
Comparing all the derivatives containing nitro groups
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 97
ISSN 2229-5518
chloro compound If, bromo compounds Ib and IIa and methoxy compound Ie, compounds Ic and IIb (para flouro) possessed significant activity in open field test when tested at the dose of 50 mg/kg body weight. It is also evident that the compound having two nitro groups at meta positions IIf exhibited highly significant activity.
It was concluded that the derivatives evaluated for be- havioral effects through open field test method, showed hyperactivity in mice which would be useful in the elevation of mood and can act as neuroleptics.
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
Compound | Dose mg / kg | Number of Square crossed in 5 minutes |
Control | - | 75.00 ± 1.41 |
Id | 50 | 96.04 *** ± 6.67 |
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10
IJSER
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com-
pared to control
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 98
ISSN 2229-5518
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
n / groups = 10

Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
100
80
60
40
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com-
20
0
Control I
pared to control
nificant differences by student’s t-test *p <0.05 and **p<
0.001.
IJSE100 R
80
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
60
40
20
0
Control I a
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
100
80
60
40
20
0
n / groups = 10
Significant different by student’s t- test: *p< 0.05, **p<0.01 as com- pared to control
Control I b
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 99
ISSN 2229-5518
100
80
60
± S. E. M. (n=10) 30 minutes after injection. Significant differences by student’s t-test *p <0.05 and **p <0.001.
1*0*0
40 80
20
60
0
Control I c
40

mean ± S. E. M. (n=10) 30 minutes after injection. Significant differ- ences by student’s t-test *p <0.05 and **p<0.001.
120
20
0
Control II
100
80
60
40
20
0
Fig 2: Show*i*n*g open field activity of Compound II. Values are mean
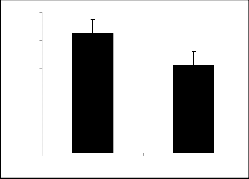
± S. E. M. (n = 10) 30 minutes after injection signification differ- ences by student’s t-test *p <0.05 and **p< 0.001.
100
80
60
IJSER
Control I d
**p<0.001.
40
20
0
Control II a
80 Fig 2a: Showing open field activity of Compound II a.
Values are mean ± S. E. M. (n = 10) 30 minutes after injec-
60 tion signification differences by student’s t-test * p <0.05 and***p< 0.001.
40
120
20 100
80
0
Control I e 60
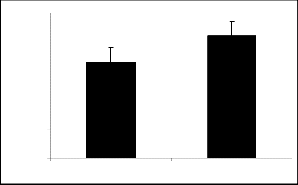
100
40
20
0
Control II b
Fig 2b: Sho*w*ing open field activity of Compound II b. Values are
80
mean ± S. E. M. (n = 10) 30 minutes after injection signification
60 differences by student’s t-test *p <0.05 and **p< 0.001.
40
20
0
Control I f
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 100
ISSN 2229-5518
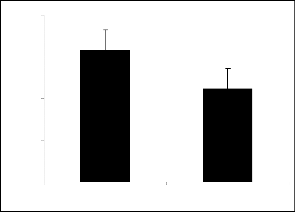
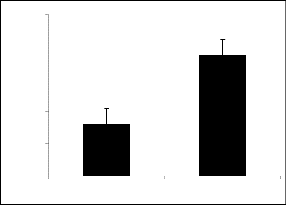
80 100
80
60
60
40 ***
40
20 20
0
Control II c
0
Control II f
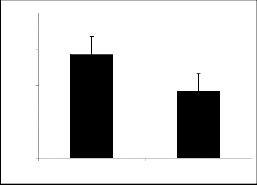
80
60
40
1 Porsolt, R.D., Anton, G., Blavet, N. and Jalfre, M. “Behavioral despair in rats: A new model sensitive to anti-depressant treatment”. Eur. J. Pharmacol. 47:
379-391 (1978).
20 2 Porsolt, .D., Bertin, A. and Jalfre, M. “Behav- ioral despair in mice: A primary screening test for ant-
Control II d depressants”. Arch. Int. Pharmacology. 229: 327-336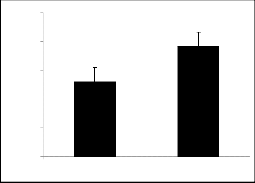
100
80
60
40
20
0
Control II e
(1977).
3 Porsolt, R.D., Lenegre, A. and Mcarthur, R.A. In: Animal models in Psychopharmacology, advances in Pharmacological Sciences. Birkhauser Verlag Basel, pp: 137-159 (1991)
4. Wi*ll*ner, P. “The Validity of animal models of de-
pression”. Psychopharmacol. 83: 1-16 (1984).
5 Shalyapina, V.G., Rakitskaya, V.V. and Rodionov, G.G. “Involvement of dopaminergic processes in the striatum during the effects of corticoliberin on the be- havior of active and passive rats”. Neurosci. Behav. Physiol., 33 (6): 629 (2003).
6 Klejbor, I., Luczynska, A., et al. “The devel- opment pattern of c-fos expression in the rat thalamus following open-field stress stimula- tion”. Pol. T Vet. Sci., 6 (3): 201 (2003).
7 Dere, E., De Souza-Silva, et al., “Connexin-
30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes”. Eur. J. Neurosci., 18 (3): 629 (2003)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 101
ISSN 2229-5518
8 Mogilnicka, E. “Increase in β- and α1 - adreno- receptor binding sites in the rat brain and in the α1 - adrenoreceptor functional sensitivity after the DSP-4-induced noradrenergic denerva- tion”. Pharmacol Biochem Behav., 25 (4): 743 (1986).
9 Hall, C.S. (1934). “Emotional behavior in the rat”. J. Comp. Psychol., 18: 385.
10 Archer I (1973) “Tests for emotionality in rats and mice: a review”. Anim. Behav.,
11 Saify, Z.S., Atia Zia and Ahmed Viqaruddin. (1996). “Synthesis and Analgesic Activity of 4- Piperidinol Derivatives”. Pak. J. Pharm. 9:1,
37-42.
12 Saeed, M., Saify, Z.S., Iqbal, Z. and Nazrul- Islam. (1997). “Studies on the effects of piper- idine derivatives on blood pressure and smooth
18 Hammann, P. (1995). In: Organic Synthesis Highlights II; Waldmann, H., Ed., VCH: New York, page No.323.
19 Laschat, S. and Dickner, T. (2000). “Stere-
oselective Synthesis of Piperidines”. Synthesis,
1781–1813.
20 Jahan, et al., 2012 Sarwat Jahan, Shamim Akhtar, Arfa Kamil, Zafar Saied Saify, Nousheen Mushtaq and M.Arif. (2012). “Anti- bacterial, Antifungal and Antioxidant Activi- ties of Derivatives of Alkyl Piperidine”. FUUAST. J. BIOL., 2(1): 29-35.
21 Sarwat Jahan, Shamim Akhtar, Zafar Saied Saify, Nousheen Mushtaq, Ali Akbar Sial, Arfa Kamil and Muhammed Arif. (2013). “Synthesis and cytotoxic activity of some de- rivatives of alkyl Piperidine”. Pak. J. Pharm.
Sci., Vol.26, No.3, pp.517-523.
IJSER
muscle contractions”. Arch. Pharma. Res.
13 Saify, Z.S., Hanifa Shahnaz, Shamim Akhtar, Moazzam Haider and D.J. Haleem. (1999). “A Study on the Effects of Some New Derivatives of Piperidine on Neurotransmitters”. Pak. J. Pharm. Sci., 12(1) 43-47.
14 Saify, Z.S., Hanifa Shehnaz, Shamim Akhtar and Kamran A. Chishti. (2001). “Cytotoxic Study of Some New Synthetic Derivatives of Piperidine”. Pak. J. Pharmacology. 18(1), 13-
16.
15 Saify, Z.S., Shamim Akhtar, M. Arif, Hanifa Shehnaz and Darakshan J. Haleem. (2005). “Neuropharmacological Estimation of Some New Morphine-Like Quaternary Phenacyl Bromo Piperidinium Compounds”. Pak. J. Pharm.Sci. 18(2): 52-54.
16 Bailey PD, Millwood PA and Smith PD (1998) “Asymmetric routes to substituted pi- peridines”. J. Chem. Soc., Chem. Commun.,
633-640.
17 Buffat MGP (2004) “Synthesis of piperidines”.
Tetrahedron, 60:1701-1729.
22 Tang G, et al., 2010) Tang G, Kertsez DJ,
Yang M, Lin X, Wang Z, Li W, Qiu Z, Chen J, Mei J, Chen L, Mirzadegan T, Harris SF, Vil- lasenor AG, Fretland J, Fith WL, Hang JQ, Heilek G and Klumpp K. (2010). “Exploration of piperidine-4-yl-aminopyrimidine as HIV-1 reverse transcriptase inhibitors. N-phenyl de- rivatives with broad potency against resistant mutant viruses. Bioorg. Med. Chem. Lett.
23 Weintraub, P.M., Sabol, J.S., Kane, J.M. and Borcherding, D.R. (2003). “Recent advances in the synthesis of piperidines and piperidines”. Tetrahedron 59, 2953–2989.
24 Yadav, J.S., Subba, B.V., Reddy, D.N., Chaya, G.G.K.S. Narayana Kumar, Naresh, P. and Jagadeesh, B. (2009). “Heteropoly acid- catalyzed aza-Prins-cyclization: an expeditious synthesis of 4-hydrooxypiperidines”. Tetrahe- dron Lett., 50, 1799-1802.
25 Yadav, J.S., Kumar, N.N., Reddy, M.S. and
Prasad, A.R. (2007). “Stereoselective synthesis
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 102
ISSN 2229-5518
of tarchonanthuslactone via the Prins Cycliza- tion”. Tetrahedron 63, pp. 2689–2694.
26 Yadav, J.S., Reddy, B.V.S., Maity, T. and Na- rayana Kumar, G.G.K.S. (2007). “A diastere- oselective synthesis of 4- azidotetrahydropyrans via the Prins- cyclization”. Tetrahedron Lett. 48, pp.7155–
7159.
27 Yadav, J.S., Subba, B.V., Reddy, D.N., Chaya, G.G.K.S. Narayana Kumar, Aravind, S., Kunwar, A.C. and Madavi, C.(2008). “Galli- um iodide/iodine as a versatile reagent for the aza-Prins cyclization:an expeditious synthesis of 4iodopiperidines”.Tetrahedron Lett. 49, pp.
3330-3334.
thorn (Hippophae rhamnoides l. ssp. turke- stanica) fruits in experimental models of de- pression”. Pakistan journal of Botany, 43(3):
1595-1599.
32 Shamim Akhtar, Muhammad Arif, Nousheen, Zafar Saeed Saify, Ahsan Ahmed, Darakhshan Jabeen Haleem and Arfa Akram. (2012). “Be- havioral and Neurochemical profile of some novel phenacyl based isonipecotamide deriva- tives”. Pak. J. Pharm.Sci. vol. 25, No.4, 705-
713.
33 Nighat Sultana and Zafar Saify. (2012). “Natu- rally occurring and synthetic agents as poten- tial anti-inflammatory and immunomodulants”. Anti- inflammatory Anti- allergy Agents Med Chem. 11(1):3-19.
28 Yadav, J.S., SubIba, BJ.V., ReddyS, G.G.K.S., ER
Narayana Kumar and Reddy, M.G. (2007).
“CeCl3 .7H2 O/AcCl-Catalyzed Prins-Ritter re- action sequence: a novel synthesis of 4-amido tetrahydropyran derivatives”. Tetrahedron Lett. 48, pp. 4903–4906.
29 Yadav, J.S., Subba, B.V., Reddy, G.G.K.S., Narayana Kumar and Swamy, T. (2007). “Ste- reo selective C-O Ring Construction”. Tetra- hedron Lett. 48, pp. 2205–2208.
30 Gao, M., Wang, M., Hutchins, G.D. and Zheng, Q.H. (2010). “Synthesis of carbon-11- labeled piperidine ring of N-[omega-(6- methoxynaphthalen-1-yl)alkyl]derivatives as new selective PET sigma 1 receptor probes”. Appl. Radiat. Isot. 68(3):459-65.
31 Ferhat Batool, Aisha Kamal, Madiha Sattar, Asad Hussain Shah, Syed Dilnawaz Ahmed, Zafar Saied Saify and Darakhshan Jabeen Haleem.( 2011) . “Evaluation of antidepres- sant-like effect of aqeous extract of sea buck-
34 Asghari Ghous and Zafar Saeed Saify(2011).
“Neurochemical and Behavioral Effects of In- dole Substituted Piperidine Derivatives”. Ka- rachi University Journal of Sciences, 39, 25-
31.
35 Nousheen Mushtaq, Saify.Z.S. , Shamim Akh- tar, Muhammad Arif, Saida Haider and Nazish Saba. (2010). “Synthesis of some novel ana- logues of 4-(1-pyrrolidinyl) piperidine and their effect on plasma glucose level”. Pak J. Pharm. Sci. 23(2): 220-223.
36 Nousheen, M., Saify, Z.S., Khalid, M.K., Perveen, S., Shah, S.T., Abdel-Jalil, R.J., Fecker, M. and Voelter, W. (2005). “5- Synthesis and biological activities of novel 4- (4´-chlorophenyl)-4-hydroxypiperidine deriva- tives”.Chem. Pharm. Bull. (Tokyo).53(1): 64-6.
37 Haleem, D.J., Yasmeen, A., Parveen, T. and Zafar A. (1994). “Enhancement of hepatic tryptophan pyrrolase activity and decrease of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 103
ISSN 2229-5518
open-field locomotion following single and re- peated administration of high doses of caffeine in rats”. Life Science, 54: 297-304.
38 Schildkraut, J.J. (1978). In: Psychopharmacol- ogy: a generation of progress, (Eds) Lipton, M.A., Di Mascio, A. and Killam, K.F. Raven Press, New York, and pp: 1223-1224.
39 Wells, K.B., Stewart, A. and Hays, R.D.(1989). “The Functioning and Well-being of Depressed Patients: Results From the Medi- cal Outcomes Study”. JAMA. 262(91): 914-
919.
40 Archer I (1973) “Tests for emotionality in rats and mice: a review”. Anim. Behav., 21: 205.
41 Shamim Akhtar, Saify Z.S, Muhammad Arif, Nousheen Mushtaq and Darakhshan J. Haleem. (2005). “Neurochemical estimations of some new quaternary Phenacyl-Bromopiperidinium compounds”. Pak. J .Pharm. Sci.18 (2):52-4.
IJSER © 2013 http://www.ijser.org