International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 719
ISSN 2229-5518
Assessment of Coagulation Efficiency of Okra Seed Extract for Surface Water Treatment
Yusuf Olabode RAJI 1*, Lawal ABUBAKAR2, Saidat Olanipekun GIWA3, Abdulwahab GIW A4
1,2,3Department of Chemical Engineering, Abubakar Tafawa Balewa University, P.M.B. 0248, Bauchi, Nigeria
4Department of Chemical and Petroleum Engineering, College of Engineering, Afe Babalola University, KM. 8.5, Afe Babalola W ay, Ado-Ekiti, Ekiti State, Nigeria
*Corresponding author
Emails: 1*yoraji@atbu.edu.ng, 2lawal.7700@gmail.com, 3sogiwa@gmail.com, 4agiwa@abuad.edu.ng
ABSTRACT: Conventional drinking water treatments are often inappropriate in developing countries, due to its high cost of its treatment, lack of appropriate infrastructures or chemicals as well as environmental factors. The present research deals with the evaluation of the treatment efficiency of natural coagulant obtained from okra seed (okra seed extract). The coagulation ability of the coagulant was assessed by the use of standard jar test experiment involving two water samples (obtained from River Rima and Goronyo Dam of Sokoto) with various coagulant doses. The coagulation capacity of the okra seed extract was measured on the basis of turbidity removal. It was found from the results obtained that okra seed coagulant was effective in removing the turbidity of surface water because the turbidities of the water samples considered were removed effectively at an optimum dose of 300 mg/L of the seed extract with optimum pH of about 7.0 from 745 NTU to 11 NTU for sample 1 and from 580 NTU to 5 NTU for sample 2. It was also discovered that the coagulant could be used to, effectively, remove the turbidity of the samples with initial turbidity of about 580 NTU to W HO standard limit of 5 NTU. Therefore, it has been discovered that okra seed extract is a very effective coagulant in water treatment.
Keywords: Water treatment, surface water, coagulation, okra seed extract.
—————————— ——————————
1 INTRODUCTION
Availability of pure drinking water has become scarce nowadays due to poor land use management. Surface water is being polluted by sewage, industrial water discharge and run off from the land, while ground water is polluted by salt water intrusion and waste dumping site. This polluted water will have to go through treatment processes before it can be circulated to the consumers for domestic use, including drinking (Anto, 2009). One of the processes of water treatment is coagulation.
Coagulation is carried out through the use of materials known as coagulants. The coagulants may be natural or synthetic. Natural coagulants have been reported to have several advantages over synthetic ones (such as alum and super plug) in that, they produce much lower sludge volume and are safe to humans. Apart from that, they (natural coagulants) are biodegradable and cost effective for developing countries since they can be locally grown and have a wider effective dosage range for flocculation of various colloidal suspensions. Actually,
there are many of them, and the one being proposed to be used in this work is okra.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 720
ISSN 2229-5518
Okra (Abelmoschus esculentus), known in many English-speaking countries as ladies' fingers, bhindi, bamia,
ochro or gumbo, is a flowering plant in the mallow family. It is valued for its edible green seed pods. The geographical origin of okra is disputed, with supporters of West African, Ethiopian, and South Asian origins. The plant is cultivated in tropical, subtropical and warm temperate regions around the world (National Research Council, 2006). According to the information available in the literature, okra has been found not only to be edible but also used as a coagulant in wastewater treatment. In fact, some researchers have used this same plant, and some other natural coagulants, for wastewater treatment in the past.
For instance, Ghebremichael (2005) used okra seed for treatment of tannery effluent and they found that okra seed was able to act as a very effective flocculent, capable of removing more than 95 percent suspended solid and 69 percent dissolved solid from the effluent. His results showed that polysaccharides (mucilage) obtained from okra and fenugreek was capable of removing 90-94% of suspended solids and 30-44% of total dissolved solids. Anto (2009) also worked on the use of natural coagulants for water treatment and discovered that the sludge produced from the use of Moringa oleifera was able to give water the turbidity of which was 20-30% better than that obtained from the use of alum.
The rapid increase in the population of the world has led to higher demand of water supply. The cost of treating water with chemicals and environmental pollution caused by sludge disposal have necessitated the idea of investigating ways to supplements or replace the use of chemical coagulants with locally indigenous plant materials.
As such, this research is contributing to this good development by using a local material (okra) for wastewater treatment through investigating the use of okra in the treatment of surface water of River Rima and Goronyo Dam.
2 METHODOLOGY
The surface water samples used for this research work of utilizing okra for water treatment were obtained from River Rima and Goronyo Dam situated in Sokoto State of Nigeria. The water samples were taken at the river banks using a set of 75 cl bottles.
The okra seeds used for the treatment of the water samples were also obtained in Sokoto town of the same Sokoto State. Before using the okra seed as a coagulant for water treatment, the oil contained in it was first extracted. The seed was prepared for oil extraction by, first, removing the shell to collect the kernel inside it,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 721
ISSN 2229-5518
and, then, crushing and grinding the collected kernels to medium fine powder using Assparo Model 900 of
pestle and mortar.
The extraction of the oil from the okra seed was carried out by adding hexane to the seed powder in an electro thermal Soxhlet apparatus. During the oil extraction operation, 10 gm of okra seed powder was weighed and put in the thimbles of the Soxhlet extraction chamber. Thereafter, 170 ml of hexane was added to the seed powder in the heating chamber. Hexane was evaporated in three cycles each for a period of 30 min to ensure complete extraction of the oil contained in the powder. Afterwards, the okra cake residue obtained from the Soxhlet thimbles was weighed and, dried and weighed again. The dried okra cake residue obtained was then used, as the coagulant, for the water treatment.
To prepare the solution of the coagulant, 10 grams of the coagulant (dry okra cake obtained after the oil extraction) was weighed and dissolved in 1000 millilitres of distilled water. The coagulant concentration was, thus, made to be 10 grams per litre of water.
Before treating the water samples, their physical parameters especially pH, conductivity, temperature, and turbidity were measured with the aid of corresponding digital meters. For instance, pH, conductivity and temperature were measured using digital pH meter, conductivity meter and thermometer whereas turbidity was measured using turbidity meter and total dissolved solids (TDS) of the samples were obtained with the aid of TDS meter.
Also, sedimentation jar test was carried out to determine the coagulation properties of the plant derived coagulant (okra). In doing this, one beaker was used as a control (blank sample) and in some other beakers were varying doses of coagulants. The jar tests were conducted using 1000 ml each of the surface water samples. In carrying out this, some amount of the coagulant (okra seed extract) was added to the water samples, and they were subjected to rapid mixing at 100 rpm for 1 minute, and, later, slow mixing step at 30 rpm for 30 min. Thereafter, the stirrer was switched off and the flocs allowed to settle undisturbed for 30 minutes. The samples for residual turbidity measurement were withdrawn using a pipette from a height of 5 cm below the surface of each beaker, and the residual turbidity of each of the samples was measured.
Furthermore, the effect of pH on the turbidity removal of the coagulant was also studied by varying the pH of the turbid water. The pH of the suspension was adjusted to the desired value by adding 0.1 M H2 SO4 solution. The turbidity measurement was carried out using Globe Instrument Turbidity meter while the pH value of the
suspension was measured using a digital pH meter.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 722
ISSN 2229-5518
After the treatment of the each of the water samples with the coagulant, its total hardness was determined by
titrating a sample of the treated water with standard 0.01 N ethylenediaminetetraacetic acid (EDTA). In this case, 100 ml of water sample was collected, using pipette, and put into a 250 ml conical flask. Then, 1 ml of buffer solution was added to the sample to maintain its pH. Thereafter, 2 drops of black T Indicator were added to the water sample, and this made its colour to become blue. The resulting sample was then titrated against 0.01 N EDTA standard solution, and when the end point, which was indicated by the occurrence of green colour, was obtained, the volume of the titre was noted and recorded, and the total hardness of the
treated water sample was thus calculated using the relationship given in Equation (1) below.

Total hardness (mg/L) = Titre × Normality of acid × 1000
𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡ℎ𝑒 𝑠𝑎𝑚𝑝𝑙𝑒
(1)
Another test that was carried out on the treated water sample was the determination of total coliform. This was
done by preparing a bacteria food sour using chocolate agar. To prepare the bacteria food, 3.5 grams of agar was dissolved in 50 millilitres of distilled water. Thereafter, three sets of 5 test tubes were arranged and to each test tube 5 ml of agar solution was introduced. Then, 0.01 ml of the treated water sample was introduced into the first set of the test tubes; 0.1 ml and 1 ml were also introduced into the second and the third set of the test tubes, respectively. The samples were then inoculated using Durham tubes. All the three set of the test tubes were incubated for 24 hours. After the 24 hours of the incubation, the level of the bacteria that fed on the coloured chocolate agar solution was examined by noting that some of the test tubes samples already turned to colourless (that is, an indication of the presence of bacteria). The result obtained was then compared with the most probable number (MPN) index and the bacteria count were read in most probable number (MPN) per 100 ml of water sample.
3 RESULT AND DISCUSSION
In this work, a solution of the coagulant was used at different doses, and their performances in the turbidity removal of the two water samples (obtained from River Rima and Goronyo Dam) are as shown in Table 1. As can be seen from the table, the initial turbidity of sample 1 was 745 NTU while that of sample 2 was 580 NTU. Upon the addition of the coagulant to the two water samples, their turbidities were found to reduce. Actually, the dosage of the coagulant was started from 260 mg/L and little reduction, which later became reasonable one, in turbidity was observed. However, at 300 mg/L of coagulant dose, the maximum turbidity removal was
achieved, and found to be 11 NTU and 5 NTU (WHO (1992) limit) for samples 1 and 2 respectively.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 723
ISSN 2229-5518
Table 1: Jar test results for turbidity removal using Habiscus exculentus (okra seed) extract
S/N | Volume of sample (ml) | Coagulant dose (mg/L) | Turbidity of sample 1 (NTU) | Turbidity of sample 2 (NTU) |
1 | 1000 | 260 | 21 | 14 |
2 | 1000 | 280 | 14 | 11 |
3 | 1000 | 300 | 11 | 5 |
4 | 1000 | 320 | 12 | 9 |
5 | 1000 | Blank sample | 745 | 580 |
It has been shown from the jar test for the okra seed that the coagulant could remove turbidity to the maximum
WHO limit of 5 NTU for a water sample with initial turbidity of not more than 580 NTU. In the sample 1 of initial turbidity of 745 NTU, the final value of the turbidity was found to be 11 NTU, when the coagulant dose was 300 mg/L. Further increase in the coagulant dose above this value (300 mg/L) was discovered to result in a re-increase in the turbidity of the sample (see Table 1).
Also, it was found that the coagulant showed a very high performance on the reduction of the turbidity of the water samples from 745 NTU to 11 NTU for sample 1 and from 580 NTU to 5 NTU for sample 2, and the use of okra seed as a coagulant did not result in any change in the pH of the water. This justifies the statements of Bratby (2006) that the use of natural coagulant in the treatment of turbid water has little or no effect on the pH of the treated water, but according to Ghebremichael (2005), the use of chemical such as aluminium sulphate as a coagulant increases the acidity of treated water.
Given in Table 2 are the results obtained from the analysis of the treated water samples. From the table, it was observed that the microbial count of water sample 1, which initially was 25 MPN per 100 ml of sample has now become 0 MPN per 100 ml of sample while that of sample 2 which was 60 MPN per 100 ml of sample before the treatment is now 0 MPN per 100 ml of sample. In addition, it was discovered from the analyses carried out that there were reasonable improvements in the values of the conductivity, the total hardness and the total dissolved solids of the water samples.
Table 2: Characteristic of treated water samples from River Rima (sample 1) and Goronyo Dam (sample 2)
using okra seeds extract coagulant at 300 mg/L optimum dosage


S/N | Parameters | Units | Untreated Sample 1 | Untreated Sample 2 | Treated Sample 1 | Treated Sample 2 |
1 | Turbidity | NTU | 745 | 580 | 11 | 5 |
2 | pH | - | 6.8 | 7.2 | 6.8 | 7.1 |
3 | Conductivity | µS/cm | 126 | 114 | 100 | 82 |
4 | Total hardness | mg/L | 24 | 14 | 24 | 17 |
5 | Total dissolve solids (TDS) | mg/L | 121 | 143 | 21 | 24 |

IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 724
ISSN 2229-5518

6 Coli forms MPN/100 ml 25 60 0 0

7 Temperature 0C 27.1 30.2 29 31
Graphically, shown in Figure 1 is the relationship between the coagulant dose and the turbidities of the water
samples. It was noticed from the figure that the highest turbidity removal for both samples were achieved when the dose of the coagulant was 300 mg/L (Figure 1). This has demonstrated that the optimum value of the coagulant dose was 300 mg/L, and any value higher than this should not be used for the treatment of these particular water samples. In addition, it is an indication that, in any case of water treatment, optimum values of the parameters involved should be used to avoid unwanted effects.
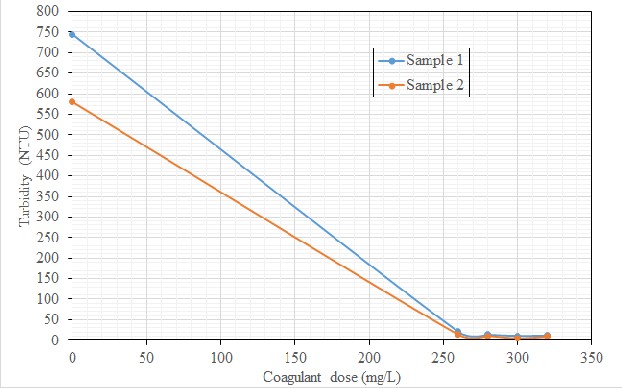
Figure 1: Plot of turbidity against coagulant dose (okra seed extract) for the two water samples


Table 3: Effects of pH at optimum dose of okra seed coagulant
S/N | pH | Turbidity Of Sample 1.0 (NTU) | Turbidity Of Sample 2.0 (NTU) |
1 | 5 | 11.3 | 5.1 |
2 | 6 | 11.2 | 5.1 |
3 | 7 | 11.0 | 4.9 |
4 | 8 | 12.1 | 5.6 |
5 | 9 | 12.7 | 6.8 |

IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 725
ISSN 2229-5518
After the highest clarities (turbidity removal) of the two water samples considered were achieved using 300
mg/L of okra seed coagulant, the pH of the water samples were varied from 5 to 9 and then treated using the optimum dose (300 mg/L) discovered. The results obtained from this treatment are as given in Table 3. From the results given in the table, it was found that the two water samples had their best clarity at the pH of 7.0. Above this value of pH of 7.0, the turbidities of the water samples were discovered to increase. In other words, above the pH of 7.0, the clarities of the water samples were decreasing. This discovery has, actually, justified the discovery of Pontius (2000) that best coagulation normally takes place at the pH of 7.0.
4 CONCLUSION
The results obtained from this research have revealed that okra seed coagulant was effective in the removal of turbidity of surface water because the turbidities of the water samples considered were removed effectively at an optimum dose of 300 mg/L of the seed extract with optimum pH of about 7.0 from 745 NTU to 11 NTU for water sample 1 and from 580 NTU to 5 NTU for water sample 2. It was also discovered that the coagulant could only be used to, effectively, remove the turbidity of the samples with initial turbidity of not more than
580 NTU to WHO limit of 5 NTU.
REFERENCES
(1) Anto, M.G. (2009). Seed as a natural coagulant for potential application in water turbidity removal.
McGraw Hill, New York.
(2) Bratby, J. (2006). Coagulants in water and wastewater treatment. London: IWA Publishers, London. (3) Ghebremichael, K.A. (2005). Water research. Journal of Water Research, 39 (11): 2338-2344.
(4) Pontius, F. (2000). Regulation for aluminium in drinking water. Journal of American Water Work
Association, 92(4): 18-22.
(5) WHO. (1992). Guidelines for drinking-water quality, Volume 1, Recommendation, Second edition. World
Health Organisation, Geneva.
(6) National Research Council (2006). Okra, Lost Crops of Africa: Volume II: Vegetables. Lost Crops of Africa
2. National Academies Press.
IJSER © 2015 http://www.ijser.org
