International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1229
ISSN 2229-5518
Author: Mary Felicia Opara Anambra State University, Uli - Nigeria feliciaopara@yahoo.com
—————————— ——————————
Abstract
The relevance of chemistry in economy, industry, health and as the center of all other sciences is well known. New
expectations arising from education reforms to improve on students’ learning outcomes and to enable them face the challenges in a rapidly growing, innovative competitive world presuppose that teaching and learning prepare students for the world of tomorrow. The traditional teaching methods preponderantly used in the chemistry classroom have proven to be ineffective in promoting students’ understanding of chemistry concepts leading to persistent failure of the subject in public examinations. Analyses of chemistry results of two African countries (Nigeria and Kenya) over a period presented in this paper reveal that students register dismal failure in the subject. This makes it increasingly difficult for students to qualify for the competitive job market or to enroll for science- related courses in the universities after secondary education. Models of constructivist teaching methods designed in this paper show how the learning theories within the constructivists’ perspective differ from the traditional approaches in promoting lifelong learning capable of enabling students acquire skills and competencies to be effective throughout their lives. The paper has implications for teachers, curriculum developers and for the chemistry students.
Keywords: Learning theories, Constructivism, Traditional teaching methods, Chemistry, Global competitiveness, Performance
Chemistry is a central science that forms the
indispensable foundation of many disciplines such as biology, physics, medicine, plant sciences, nuclear chemistry, engineering, geology, cosmetics, and environmental science [29].
Disciplines within chemistry are traditionally grouped by the type of matter or kind of study in question. This includes the study of matter; the study of chemical processes using physical concepts such as, the analysis of material samples for their properties or characteristics. Chemistry protects and preserves our health, culture and heritage. Chemistry provides important understanding of the world. It is a practical science that impacts on our day-to-day living. The life we live is chemistry, the water we drink, the energy we use to cook and materials we use at home are products of chemistry. Chemistry impacts on the dynamism of our intra and extra movements. It is a science that lives at the heart of many matters in the society. Thus, the knowledge of chemistry in ever increasing innovative world is a sine qua non because achievement in chemistry is crucial for ensuring economic competitiveness. This
implies that lack of conceptual understanding of chemistry content beginning from the secondary school level may impact negatively on students in a world that is becoming more global, innovative, dynamic, competitive and requiring quality and efficiency in the workforce. It is therefore critical to continue to address the issue of students’ persistent poor performance in chemistry.
Research has shown that students in
developing countries such as Nigeria and Kenya who register chemistry at the West African Secondary School Certificate Examinations (WASSCE) and Kenya Secondary Certificate Examinations (KSCE) perform poorly [21]; [22]; [23] despite world-wide attention on improving students’ learning outcomes.
IJSER © 2013 http://www.ijser.org
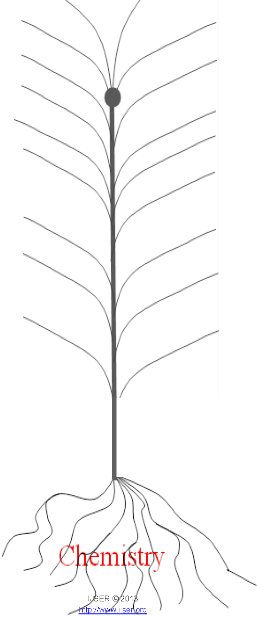
Figure 1: Chemistry as Central Science (Branches of
Cht!ffi'JJ;fr\\;i'afrtl l-8fat£ia. E$finl i;t)eering Research, Volume 4, Issue 10, October-2013
ISSI'T2229-5518
1230
Quantum Chemist!y Jevvelty Chemishy

Phyto- Chemist!y
.Analytical Chernistty
A lvlechano Chemist!y.
ltrunune - Chemistry
Geo-Chemist!y

-- Nuclear Chemist!y
.
enust!
y . ) L j
Agriculbml exle11sicn
![]()
SoilScien::e Neuro - Chemist!y
Engineering Chemist!y
Chmrical e m1g
ChenUcal Bio Biontechanics Mac10nclerularchmlistry
Agro- Chemistty Clinical Chemislly

Biochemist!y
lecn·o-Chemistty
Environmental Chemist!y
O:eanic el\gilteeru
lvieclicin al Chemi stty

Inorganic Chemistty
Organic Chemist!y
Solid state Cremistry Euvilomt\Olliill Scien:e J.JfurilJg
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1231
ISSN 2229-5518
Analysis of results for six consecutive years as shown in the graphs below provides evidence of students’ performance on the subject. In Kenya, the mean achievement scores of students in chemistry at the KSCE from 2005 to 2010 were 26.99, 24.78,
25.17, 22.50, 18.99 and 24.71 respectively. In
Nigeria, from 2005-2010 analysis of results of students who sat for WASSCE from 2005 to 2010. showed that 37.28%, 50.65%, 45.11%, 46.16%,
43.69% and 50.70% respectively passed at credit level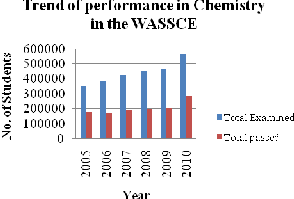
Figure 1: Trend of performance in Chemistry in the WASSCE
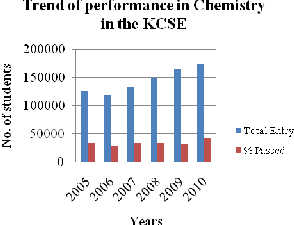
Figure 3: Trend of performance in Chemistry in the KCSE
This level of pass cannot foster global competitiveness or promote students’ headway for admission into the Universities or Higher institutions for science related subjects that need chemistry as a
prerequisite. Failure also shows that secondary school students are not being prepared for the global demands in the current era. A few studies proffered reasons for the persistent failure, [2]; [3] attributed the failure to lack of adequate teaching resources while [1] viewed students’ attitude as a contributing factor. In a recent study carried out by [36] on Ethiopian University students, 82% and 80% of students respectively ascribed their lack of interest and motivation in chemistry to the teacher and teaching method. Similarly, [5] asserted that the poor performance was due to teachers’ emphasis on content coverage and teachers’ lack of interest to try new teaching methods.
In Kenya, concern about the persistent poor performance in science and mathematics led to the mounting of SMASSE (Strengthening Mathematics and Sciences in Secondary School Education) project. The project was organized by the Ministry of Education Science and Technology (MOEST) in collaboration with the Government of Japan through Japan International Cooperation Agency (JICA). The aim of the project was to provide in-service training for mathematics and science teachers to help them improve on their pedagogical content knowledge (PCK) and teaching methodology. Thus, the SMASSE initiative was based on the need for effective classroom practices and a shift from teacher- centered teaching methods to student-centered and activity-based methods. A similar trend to the Kenya experience was that in 2009 the Federal Government of Nigeria through the Minister for Information broadcasted the implementation of “re-branding” to be applied to all sectors of Government including education. The aim of re-branding was to adopt new and better ways of demonstrating responsible lifestyle that will foster the achievement of the country’s vision 2020 in sustainable human development. In line with this, education reform agenda was campaigned with the aim of revamping science, technology and mathematics while imbedding innovative systems for ameliorating achievement, enterprise, development and economic growth [35]. In the view of [19] there was also a need to re-brand the country’s education sector through teaching methods. One can therefore affirm that teaching methods remain a critical area of concern to be persistently addressed until success is achieved.
Studies have shown that about 51% of practicing teachers in Nigerian secondary schools are not professionally qualified to be in the classroom [30] and [26] found that a major defect in Nigerian science teaching is lack of application to real world. Further studies confirmed that most science teachers do not possess the prerequisite knowledge needed for
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1232
ISSN 2229-5518
activity-based learning [20]; [13]. Hence, the prevalent teaching method in Nigeria and Kenya is talk and chalk approach [17]; [15]; [9]: [10]. It is in the light of the evidence provided that this paper sought to design chemistry teaching models based on the learning theories to enhance teaching skills that may not only improve students’ achievement if appropriately adopted in several innovative ways, but may well foster students’ acquisition of 21st century competencies that could enable them face the challenges in a rapidly growing competitive world.
1. Concept of Learning Theory and models:
A learning theory endeavors to describe how children
learn. A learning theory helps us understand the process of learning [18]. Learning theories play major role in instructional design models. Instructional design model conveys the entire idea of how to organize applicable instructional or pedagogical representation for achievement of instructional goals. Instructional models are strategies on which the styles or methods of teaching are based. According to [7] instructional models prescribe how combinations of teaching strategies should be incorporated to produce a structure of instruction. Models help learners to understand scientific concepts through visualization and the simplification of concepts or problems. Effective instructional models are based on the learning theories.
2. The Learning Theories: Four major schools of learning theories that exist to date are: Behaviorism, Cognitivism, Constructivism and Connectivism [18]. Behaviorism and cognitivism belong to internal or acquisition theories while contructivism and connectivism belongs to external or participation theories [31].
The internal acquisition theories have overtime advanced the idea that learning is a passive reception of information, to learning as an active search whereby learners build their schemas by assimilating new information into existing schemas of knowledge [21]; [38]. Participation theories on the hand give prominence to active participation of learners within a social group or context. This theory places strong emphasis on ‘doing’ and validates science as a process of doing. This paper deal with some aspects of the learning theories dealing with internal and external theories. [31] recommended that for effective teaching, a combination of the theories should be employed to take cognizance of students’ diverse needs. Cognitive learning theory focus on the mental construct’s and organizational patterns that an individual develops in the process of formation of new reasoning patterns in response to his/her inadequacy in using present reasoning patterns to
cope with a demand [16]. In the course of the formation of new reasoning patterns, the individual actively engages his/her internal mental processes to combine new experiences with prior experiences and to generate logical operations.
Research has shown that knowledge is stored as a network of concepts in the brain of the leaner and that learners construct knowledge by making connections between new information and their conceptual network or mental structures [27]. The constructivist focuses on learners’ construction of knowledge in their own understanding. From the standpoint of the constructivists, learners develop shared-meaning through the process of interaction with phenomenon, within a social context as they construct new knowledge. As far as the constructivist is concerned, learning is something that is done by the learner and not something imposed on him/her from outside, hence, the doing aspect of the external theories. In the view of [19] “learning can be thought about as a process of conceptual change in which faculty or incomplete models are repaired” p161. Conceptual change occurs during the process of dis- equilibration or cognitive conflict.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013
ISSN 2229-5518
1233
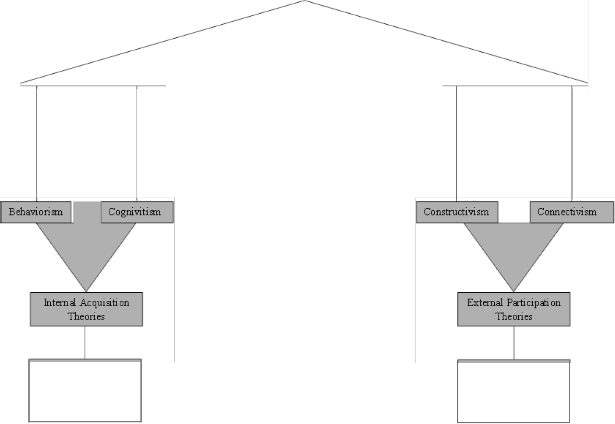
Figure 4: The Learning Theories
Learners assimia! tes newinformction into existing schemes of knowledge
Emphasizes active p <rticipation of le<mers in groups
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1234
ISSN 2229-5518
According to [26], accommodation which is the changing of one’s own thinking in order to strive to equilibrium includes analysis of a situation to locate source of conflict and the formation of new hypothesis to plan an attack [11]. Prior to accommodation, the individual’s mind was in a state of equilibrium but with the assimilation of new data the mental structures become inadequate to tackle the new situation and so must undergo process of change. Change in thought unit also gives rise to change in organizational pattern. The process of organization further entails changing the original mental structures. The end result of equilibration is increase of knowledge and a deeper understanding of experience encountered or of content in the area of science under investigation.
Social constructivism is established on the
work of [26], [35], Bartlette, Bruner, Rodoff and Gestalt psychologists, who theorized that learners’ understanding is both individual and social [39]. Two broad areas of constructivism exist: they are psychological and social constructivism. Social constructivism which is of social learning is deep- rooted in Vygotsky’s theory. Social constructivism emphasizes the importance of relationship between students; the student and the instructor in the learning process. The role of learners’ active participation as a team, shared responsibility [39] and social interaction in fostering critical and creative thinking and understanding of science concepts has eminence in social constructivism. Interaction in groups while carrying on hands-on activities provide learners opportunity for negotiation of meaning and arriving at consensus- an important mechanism in the equilibration of discrepancy and disagreement. [35] also believed that learning cannot be separated from the social and cultural settings in which it takes place.
Schema Theory: An attempt to explain how new information is encoded in the long term memory is the realm of the schema theory. Schema theorists opine that concepts are best understood after foundation of concrete and relevant information has been established [32]. The theory suggests that prior knowledge can expedite transfer of a learning task. Hence, for students to gain understanding and perform tasks in chemistry effectively, their prior knowledge must be provoked. Information processing model-elaboration processing strategy stresses the links between the information stored in long term memory and the new information. Therefore, the awareness of the relationship between concepts is very significant to cognitive teaching and learning in the classroom [13]. For meaningful learning to occur, students must be able to relate new
knowledge (concepts or propositions) to what they know already. Meaningful learning implies that the learner fully comprehends the concept being learned and that the individual knows how that specific concept, ideas or facts relate to other stored facts [4]. Meaningful learning which the learning theories tend to achieve leads to deeper learning. Deeper learning is vital strategy through which students find meaning and understanding from course material and experiences [37]. With deeper learning students gain the competence to transfer knowledge to real life situations.
Traditional Teaching and Modern Teaching
Methods
The traditional teaching methods which
comprise expository, discussion and demonstration approaches are teacher-centered. Expository instruction has been criticized for placing little emphasis on thinking. It has been described as a “cookbook” nature of learning. Traditional instruction which is heavily driven by ‘teacher-talk’ involves the transmission of knowledge by the teacher to passive listeners. In science classroom where the traditional approaches dominate, little learning takes place [35] as the learner’s goal is to regurgitate the information or procedure as prearranged by the teacher [8]. A supposition which is fundamental in the teaching method is that students are ‘empty urns’ into whom teacher is expected to pour knowledge. The teacher determines the outcome of the learning process and the learner is not challenged to create or critically contest teacher’s results. The design in traditional approaches is such that learners spend more time in finding correct answers rather than critically thinking out how to construct their own meaning of scientific concepts. With emphasis on content coverage learners have little or no time for resolution of cognitive conflict and for interaction in groups where they can explain their own position on the learning process as they explore, elaborate and carry out hands-on activities. Several studies that compared the traditional teaching methods (TTMs) with constructivist methods tended to prefer the latter for stimulating conceptual change and meaningful/deeper learning [34]; [6]; [9], [30]. Figures 5 and 6 show the features of Traditional Teaching Methods and Constructivists Teaching Methods.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1235
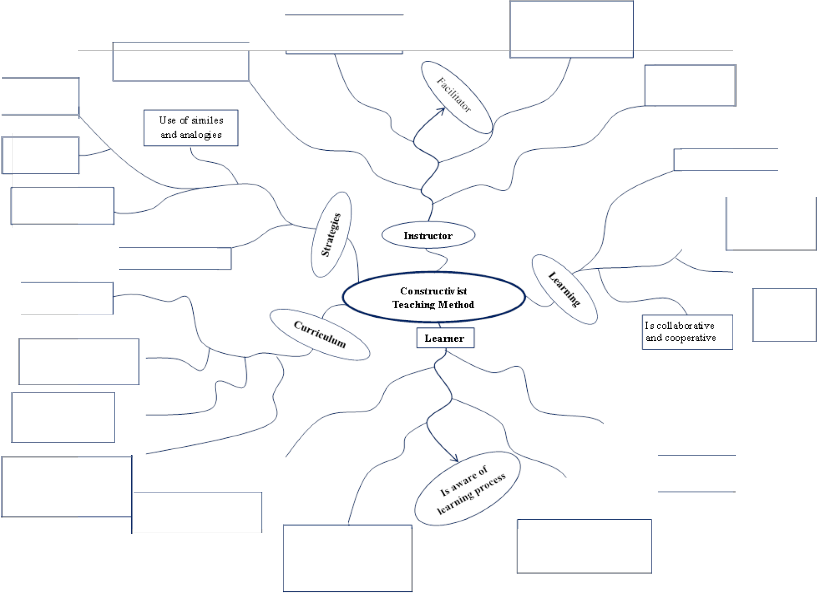
Figure 5: Characteristics of Constructivist Teaching Method
Hypothesis testing
U ses new inform ation on students' experiences
Encourages Critical and ere ative thinking
Guides, Validates, Motivates, Suggests and Models
Activates prior knowledge
Informative
feedback Is Student- centered
Use of @:'aphic
organizers Promotes metacognitive
strate@es
Quality homework
Has relevance
to r eal life
E nhances self regulation
Students have opportunities to resolve cognitive conflicts
Task cycles match development learning cycles
Align resources & ICT goals for maximum motivation of students & instructional goals
Is actively engaged in hands- out activities
Constructs know!edge by connecti ng new informati on in existing
scheme
Constructs their own understanding of concepts
I s in control of learning
IJSER 2013
Motkftfuundddcol'di'iagit!•®afids R.f&le.olly Vol ume 4, Issue 10, October-2013
Figlift11:2WF-Utgetian Mode l: Illustration of a typical lesson
1236
![]()
![]()
The following questions guide the students in their asse$ment of understarcling of conce pts, skills, etc.
i. What did YJU understand by the tenn \W.ter of crystallization?
ii. What volume of sodium h)ldroxide NaOH \W.S needed to neutralize 6.25 cm3 ofvinegar?
iii. Neutralization of an acid by abase in a laboratory norma lly takes place in a conical flask and is visible by means of colour change in an indicator. The scale of ti!ration is in amounts of 25
to 50 cmJ
![]()
![]()
A student collects the following daIa for the purpose of calibrating a dropper pipette in the laboratory. The calibration involves detennining the volume of a drop of wale r delivered in the pipette. Review the daIa and answer the following questions:
• Are the two quanhlles m the table proportional? Ho wdo you know?
• Which proportional relationship or ratio is most useful to the student in calibrat ing the pip:ltte?
![]()
Carry out the titration bet en hydrated sodium caroonate and 0.5M h)ldrochloric acid. Choose the suitable irclicator. You are supplied with 40g of washing soda crystals to
prepare a solution of 1OOOc m3 Carry out their ti!ration and
record your readings.
Group B in the class is supplied with vinegar, 0.1 M sodium h)ldroxide . Given 25 cm3 of vinegar, pre pare a solution of
100 cm3 and titrate against the 0.1 MNaOH.
i. How many times did you dilute the original volume of
vinegar given to you?
ii. What is the concentration of ethanoic acid in the vinegar? iii. What is the percentage of e thanoic acid in the vinegar? iv. Determine the quantityof\W.ter present in the hydrated NalC03 from the mass ofh:y:irated Na2C03 and the anh)drous NaC0 3?
v. From the latter what is the percentage ofwaterof
cr)61allization in the (original) hydrated sodium trioxocaroonate IV .
vi. What is the fonnula of hydrated NaC0 1with
unknown number of molecules of \W.ter present?
vii. Establish a relationship between mass of anh:y:irated
![]()
NalC03 and the relative molar masses'?
Students interact in groups and explain the proce dures in solving the problems. Teacher facilitales and corrects misconceptions (if any).
IJSER <S>2013
At)))tlratus: Washing soda (Sodium calbonat. 10 H20), Vinegar, burettes, pipettes, conical flasks, indicators (meth)d. orange and phenolfhthalein), funnels, white tiles, 0.5Mh)drochloric acid and O.lM sodium h)ldroxide, volumetric flasks, weighing balance,
\W.ter. Alter titration teacher leads them to the next phase by asking them the following questions?
Is iI possible to calculate the percentage of \W.ter of crystallization in the sodiurn
trioxocaroonate IV ? HoW?
How could the percentage of ethanoic acid in vinegar be detennined?
What is the equation 10 r the reaction between ethanoic acid and sodium h)ldroxide?
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1237
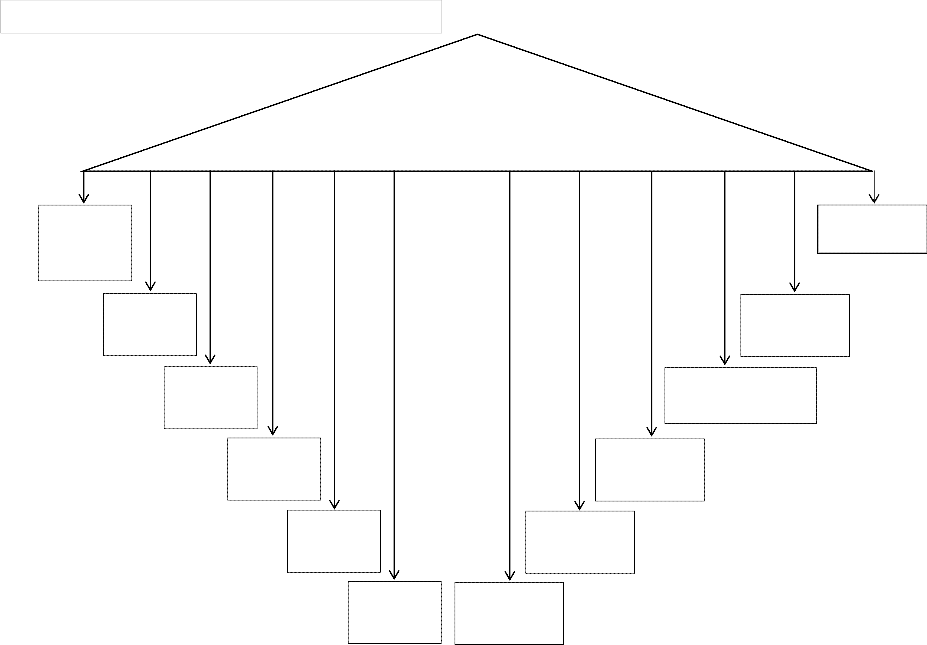
Figure 6: Characteristics of Traditional Teaching Method
Teacher fully owns the responsibility of teaching in classroom
Lesson is competitive
Teacher explains, asks questions and students write
Content is considered more important
Teacher is in full control of the learning environment
Student’s misconceptions are not challenged. New formulas or theories are rarely based on existing knowledge or experience
Teachers’ decisions are supreme
Content is not related to real life
in any way
Teachers causes learning to occur
Learning stops in the school
Students are passive
listeners
Student memorizes concepts and copies notes
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1238
ISSN 2229-5518
Instructional Design Model: An instructional design model is a framework on which teachers can base their instruction in order to improve on the learning process to foster student’s understanding of scientific concepts. Instructional research models can also make difficult science concepts easier to conceptualize. It is possible to design several models based on the learning theories but this paper designed models based on [26] and [35] theories, that is, the inquiry model and another based on the schema theory (information processing model).
Information Processing Model: Information processing model has its foundation on the schema theory. [11] translated the model into an instructional design constituting phases of learning.
Gagne’s (1985) Phases of Learning
Table 1: Instructional Design Model
Internal process | Instructional event | Action example |
Reception expectancy | 1. Gaining attention 2. Informing learners of the objective | • Use abrupt stimulus change • Tell learners what they will be able to do after learning |
Retrieval working memory | 3. Stimulating recall of prior learning | Ask for recall of previously learned knowledge or skills |
Selective perception | 4. Presenting the stimulus | Display the content and distinctive features |
Semantic encoding | 5. Providing learning guidance | Suggest meaningful learning |
Responding, reinforcement and retrieval. | 6. Eliciting performance 7. Providing feedback 8. Assessing performance | Requires additional learner performance with feedback |
Retrieval and generalization | 9. Enhancing retention transfer | Provides varied practice and space |
Seeing that students have for many years registered poor performance in chemistry despite the research efforts aimed at improving students performance, it has become imperative for teachers to adopt models that are based on sound and tested theories. This study therefore examines the extent to which the models given in this study will impact on student’s performance in chemistry.
Research Questions: To what extent will the
application of models based on the learning theories affect student’s performance in chemistry as against those taught using the traditional methods?
How does gender impact on students’ performance in chemistry using models founded on the learning theories and students taught using traditional methods?
Research Hypotheses: The mean performance scores of students taught using models founded on the learning theories and those taught using the traditional approaches will not differ significantly.
Research Design: The study was a quasi- experimental pre-test posttest design. The non- equivalent control group design was used because intact classes were involved. When administrative decisions such as such school regulations prevent random assignment of subjects to treatment and control groups it is advisable to use non-equivalent control group design.
Target Population: The population comprised all chemistry students in Senior Secondary Class II of two Special Science Schools in Anambra State, Nigeria.
Method: The study sample comprised two hundred and twenty four (224) SS II students from one male and one female Special Science Schools in Anambra State - Nigeria. Purposive sampling was used to select all the students in SSII class because chemistry is a compulsory subject for all students in Special Science Schools. These schools were double streamed. Thus, four intact classroom groups were randomly assigned to different classes of the same school. The treatment group was made of 112 subjects (56 boys and 58 girls). The control group comprised (56 boys and 57 girls). Simple random sampling was used to select the responses of 56 boys and 56 girls for the treatment and control groups respectively to ensure equal replication of subjects. The sample for which data was complete was 224 subjects (112 female and 112 male) for the treatment and control groups respectively.
Instrument The instrument for the study was Chemistry Achievement Test (CAT) based on syllabus for SS class II with additional tests on the relevance of chemistry, topics precluded in the syllabus. The test was a 20-item objective question on organic chemistry and quantitative chemistry.
Reliability of the instrument: The instrument was subjected to trial testing using 40 chemistry
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1239
ISSN 2229-5518
students in a school different from the Special Science Schools used in the study. The scorer reliability estimate using Pearson- Product moment correlation was found to be 0.84.
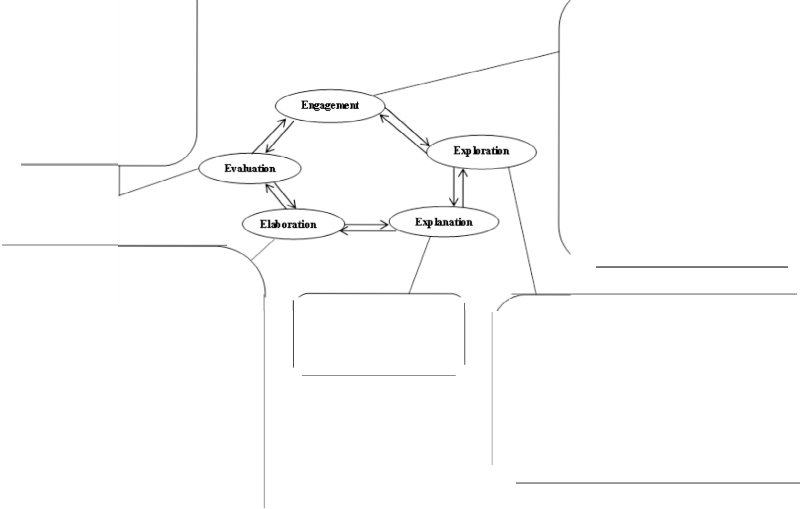
Instructional Procedure (Experimental Group): The following instructional event designed for teaching a topic in organic chemistry is a model from the schema theory based on Gagne’s phases of instructional model. The method used was the creation of typical lesson intended to implement the principles of the learning theory depicting the information processing model. The teacher’s role was that of a facilitator whose duty it was to provoke students to think, create and take responsibility for the learning process. The reception expectancy stage informed students of what was expected of them by the end of the activities. At the retrieval working memory phase, questions were posed that elicited recall from the memory of learners’ previous knowledge. The ability of learners to select material based on concepts under study was tested. Activities in the semantic encoding stage were an attempt to encrypt new knowledge with previous knowledge so that knowledge can be stored both in the short term and long term memory. Activities merged chemistry concepts under study and leaner’s natural setting. Responding retrieval linked evaluation, further explanation, exploration and homework task that support lifelong learning cycle that will enhance retrieval and sustainable education.
Table 2: Instructional Event: Lesson on polymerization
Reception Expectancy | Specific objective: By the end of the lesson students should be able to explain: (i) the terms monomer, polymer and polymerization. (ii) Mention types of polymerization. (iii) Make a list of products of polymerization. (iv) Apply knowledge to environmental issues. |
Retrieval Working Memory | (i) What is a molecule? (ii) Give example of a small and large molecule in nature. (iii) What does term poly mean? Now proffer one word to depict a large molecule. (iv).What type of reaction do unsaturated hydrocarbons undergo? (v). Mention 4 unsaturated compounds that exist in nature. |
Selective Perception | Teacher had prepared nicely and delicious pot of indomie for experiment, the left over will be |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1240
ISSN 2229-5518
Discuss. (vi) How can litter cause serious health problems? (v). Paper and plastics represent a huge threat to the environment and interfere with the ecosystems. Describe a typical scenario of what happens on a very rainy flood day as per highways,
farms, gutters and drainages.
The traditional group was taught using talk-chalk method and teacher demonstration as students’ listened copied notes and followed the teacher’s step- by-step procedure.
Data Presentation
The Test of Assumption Between the Analysis of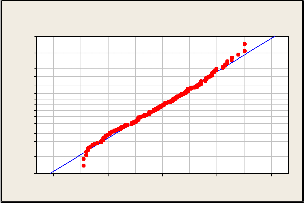
Variance (ANOVA) – the normality between the dependent variables is shown in Figure 8 below.
The table 3: Means and standard deviation of students’ scores in post-treatment CAT (by treatment by Gender).
Normal Probability Plot of the Residuals
(response is Score)
99.9
99
The table shows that the mean performance score of
95
90 the treatment group is 66.12 as against 42.22 for the
70 control group. Thus, the experimental group has a
60
50
40
30
20
10
5
1
0.1
-50
-25
0 25 50
Residual
Figure 8: Test of Assumption between the Analysis of Variance (ANOVA) – the normality between the dependent variables
The graph shows that the data follows a normal distribution.
Research Question 1: To what extent will the application of models based on the learning theories affect students’ performance in chemistry as against those taught by traditional methods?
higher mean score than the control group.
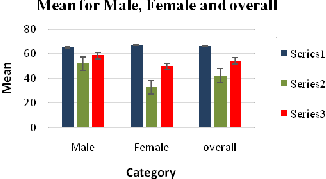
Figure 9: Showing the Mean Scores – Experimental and Control Groups; Gender Scores
Series 1 is Experimental group
Series 2 is the control group
Series 3 is the overall
To determine the level of significance of the mean scores ANOVA was carried out.
Ho1 The mean performance score of students taught using models founded on the learning theories and those taught using the traditional method will not differ significantly
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1241
ISSN 2229-5518
Table 4: One-way ANOVA: Score versus Group
Table 7: Comparison of the performance (boys and girls) taught using the models (Mean and SD)
Level | N | Mean | SD |
Boys | 112 | 58.46 | 12.94 |
Girls | 112 | 49.87 | 25.44 |
S = 16.79 R-Sq = 33.82% R-Sq(adj) = 33.52%
The p-value is 0.000 which is less than 0.05. Therefore at 5% level of significance, the null hypothesis was rejected. Hence, teaching method had a significant effect on the mean score of students.
Individual 95% CIs for Mean Based on Pooled SD
Table 5: Mean Scores and Standard Deviation of the Control and Experimental Groups
Level | N | Mean | SD |
Control | 112 | 42.22 | 18.66 |
Experimental | 112 | 66.12 | 14.68 |
224 | 54.17 | 16.67 |
The mean score of the experimental group (66.12) was higher and significantly different from that of the control group (42.22).
To determine the significance of gender on the mean performance scores of students taught using the models, Table 6 below reveals the results.
Gender does not have a significant impact on students mean performance scores.
One Way ANOVA: Scores versus Gender
Table 6: Determination of Significant Level of gender on the mean performance scores of students
Source | DF | SS | MS | F | P |
Gender | 1 | 4131 | 4131 | 10.14 | 0.002 |
Error | 222 | 90408 | 407 | ||
Total | 223 | 94540 |
S = 20.18 R-Sq = 4.37% R-Sq (adj) = 3.94%
The p-value is 0.002 which is less than 0.05. Therefore at 5% level of significance, the null hypothesis was rejected. Therefore, gender had a significant effect in the mean score.
Individual 95% CIs for Mean Based on Pooled SD
Pooled SD = 20.18
The mean score of boys was better (58.46) and significantly different from that of girls (49.87).
Discussion
Evidence from the findings of this study showed that
teaching that was based on the learning theories significantly impacted on students’ performance in chemistry. The findings are in agreement with [33]; [6] who opined that constructivist methods fostered conceptual understanding more than the traditional methods. In addition, when students are involved in learning process, they develop competencies needed for knowledge transfer [36]. The stages in Gagne’s steps of learning were meant to enhance the encoding of concepts in both short-term and long-term memory. That boy performed better than the girls seemed to agree with [24] who asserted that boys perform better than girls in the sciences. However, since girls in the experimental group had a higher mean score than girls in the control group, the teaching method enhanced the performance of both boys and girls.
Conclusion
In a world rapidly needing young people who are
capable of decision-making through their capacity to use direct and inverse proportional reasoning, teaching methods which encourage memorization have become obsolete. Persistent failure in Chemistry among African chemistry students suggests that African leaders need to pay more attention on the quality and standard of education because educational achievement is the hallmark of any nation and the key to success of democracy and economy. The reality of this fact comes to light when students cannot gain entry into the Universities or institutions of higher learning for those disciplines which require chemistry as basic entry qualification. Finally, teachers must prepare students for the demands of the global business community which they must face after school – hence, the need to base teaching methods on sound evidence-based theories.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1242
ISSN 2229-5518
References
[1] Adesoji, F. A. & Ogunni, A. M. (2012). Students’
attitude indices as predictor of learning outcomes in chemistry. British Journal of Arts and Sciences 8 (11), 174- 182.
[2] Arokoyu, A.A., & Ugonwa, R. M. K. (2012).
Assessment of resource availability for chemistry instruction in the secondary schools in Rivers State. Journal of Emerging Trends in Educational Research and Policy Studies, (JETERAPS), 3 (3), 346-351
[3] Asiyai, R. I. (2006). An appraisal of the adequacy of the physical resources for teaching chemistry in secondary schools. In Uchenna Nzewi, 47th Annual Conference Proceedings if ths Science Teachers’ Association of Nigeria. Resources for Science Technology and Mathematics Education.
[4] Ausubel, D.P. (2000). The requisition and retention of knowledge. A cognitive view. Kluver Academic Publishers
[5] Bamidele, E. F. & Olyede, E. O. (2013).
Compararive effectiveness of hierarchical, flowchart and spider concept mapping strategies on students’ performance in chemistry. World Journal of Education. 3 (1),
66-76.
[6] Bishop, R. & Berryman, M. (2006). Culture Speaks : Cultural Relationships and Classroom Learning. Wellington: Huia Publishers.
[7] Braxton, S., Bronico, K. & Looms, T. (1995).
Instructional design methodologies and techniques
[8] Caprio, M. W. (1994). Easing into constructivism, connecting meaningful learning with student experience. Journal of College Science Teaching. 23 (4), 210-212.
[9] Derebssa, D. S. (2006). Tension between traditional and modern teaching – learning approaches in Ethiopian primary schools. Journal of Cooperation in Education 9 (1),
123-140
[10] Ezeliora, B. (2004). Motivating secondary school teachers to face the challenges of the third millennium. Journal of Science Teachers Association of Nigeria 39 (1&2), 14-18.
[11] Fowler, L. S. (1980). An application of Piaget’s theory of cognitive development in teaching chemistry:The learning cycle. Journal of Chemical Educator 57 (2), 135-136
[12] Gagne, R. M. (1985). The conditions of learning and theory of instruction. New York : CBS College Publishing
[13] Jayapraba, G., Kanmani, Moi University (2013).
Matacognitive awareness in Science classroom of higher secondary students. International Journal on New Trends in Education and their Implications 4(3), 49 - 56
[14] Johnson, K. (2004). The role of paleontology on teachers’ attitude towards inquiry science. Retrieved on-line, 15th April, 2013. http://novationsjournal.org
[15] Kamau, D.M. (2012). A study of the factors responsible for poor performance in chemistry among secondary school students in Maagwa District, Kenya. Unpublished M.Ed thesis Kenyatta University, Kenya
[16] Karplus, R. (1977) Science teaching and the development of reasoning. Journal of Research in Science Teaching. 14 (2),169-1
[17] Kurumeh, M. S., Omenka, J. E., Mohammed, A S. (2013). Re-branding mathematics : An approach to enhancing students’ performance in mathematics in Anambra State, Nigeria. Greener Journal of Education Research. 3 (1), 039-045
[18] Lepi, K. (2012). A simple guide to a 4 complex learning theories. Retrieved on-line from Edudemic.com: http://www.edudemic.com/2012/12/a-simple- guide-to-4-complex learning theories/
[19] Micheal, J. (2006). Where’s the evidence that active learning work? Advances in Psychology Education 30, 159-167
[20] Nwosu, A. A. (2004). Teachers’ awareness of creativity related behaviours science classroom. Journal of Science Teachers’ Association of Nigeria 39 (1&2),22-26
[21] Obamanu, B. J. & Ekenobi, T. N. (2011).
Analysis of learning outcomes in chemistry among SSII students in urban and rural setting
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1243
ISSN 2229-5518
using concept mapping techniques. Journal of
Educational and Practice. 2 (4), 148-154
[22] Ogwe, J. C. A., Odhiambo, J. & Kibe, S. (2008).
Impact of SMASSE on students’ capacity through improved teaching and learning in the classrooms. Academia edu 2,668.
[23] Okebukola, P.A. (2007) Students’ performance in practical chemistry : A study of related factors. Journal of Research in Science Teaching 24 (2), 119-126.
[24] Okeke, E. A. (2002). Gende, Science and Technology for Africa : A challenge for education. The 2000 Rama Mental Lecture. Radellife College.
[25] Oludipe, D. I. (2004). The importance of instructional materials on the successful implementation of the core curriculum for the integrated science at the junior secondary school level. Knowl. Rev. 9 (1),17-24
[26] Piaget, J. (1977). Equilibration of cognitive structures. New York : Hayes and Row
[27] Peterson, P., Fennema, E. & Carpenter, T. (1988). Using knowledge of how students think about mathematics. Educational Leadership, 46, 42-46.
[28] Price, W.S. & Hill, J.O. (2004). Raising the Status of Chemistry Education. In RCS. Retrieved 11/09/2013, from http://www.rsc.org/Publishing/Journals/RP/iss ues/2004_1/raising.asp.
[29] Salman, M. F., Olawoye, F.A. & Yahaya, L.
A.(2011). Education reforms in Nigeria : Implications for girl-child participation in science, technology and mathematics (STM). Education Research Journal 1 (1), 1-8.
[30] Saville, B.K., Zinn, T. E.,Neef, N. .A., Norman, R.V. & Ferreri, SD. J.(2006). A comparison of interteaching and lecture on the college classroom. Journal of Applied Behaviour Analysis. 39, 49-61
[31] Sfard, A. (1998). On two metaphors of learning and the dangers of choosing just one. Educational Researcher. 27 (2), 4-13.
[32] Schallert, D. L. (1982). The significance of
knowledge : A synthesis of research related to schema theory. In Wayne Otto & Sandra White (eds) Reading expository material. Retrieved on-line 30th April, 2013 from philpapers.org/rec/SCHTSO-19
[33] Taurina, T. (2007). Secondary school teaching and Maori student achievement in science. MAI Review Intern Research Report. 11 p1-12
Retrieved on-line 11th July 2013 from http://www. Review.mai.ac.nz
[34] Ukonu, J. O. J.(2010). Re-branding Nigeria for sustainable science and technological development and transfer. Owerri, Nigeria. Ultimate Printing and Publishing
[35] Vighnarajah, L., Luan, W. and Abubakar, Kenya (2008). The shift in role of teachers in the learning process. European Journal of Social Sciences. 7(2), 33 – 41.
[36] Vygostky, L. S. (1975). Mind inSociety : The development of higher mental processes. Cambridge M. A: Harvard University Press.
[37] Warburton, K. (2003). Deep Learning and education for sustainability. International Journal of Sustainability in Higher Education
4, (1), 44-56.
[38] Woideammanuel, M., Atagana, H. & Engida, T. (2013). Students’ anxiety towards the learning of chemistry in some Ethiopian Universities. AJCE 3(2), 28-3
[39] Woolfolk, A. (2010). Educational Psychology.
Eleventh edn. Pearson Education, Inc.
IJSER © 2013 http://www.ijser.org