International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 138
ISSN 2229-5518
Apparent molar volumes and viscosity B- coefficients of sodium salicylate in different
solvent systems at different temperatures.
Arun B. Nikumbh , Ravindra C. Bhujbal
Abstract-Apparent molar volumes (фv) and viscosity B-coefficients for Sodium salicylate solutions in NS (Normal Saline), DNS (NS with dextrose), RL (Ringer lactate) and pure water solvent systems have been determined from density (ρ) and viscosity (η) measurements at
298.15 to 313.15 K using a bicapillary pycnometer and Ubbelohde viscometer respectively. The data were analyzed using Masson’s
equation to obtain limiting apparent molar volumes (фv°) and experimental slope (Sv). The Jones-Dole equation have been used to analyze viscosity data to obtain viscosity ‘A’ and ‘B’ coefficients. The drug interacts with various ions or molecules or biological membrane present in the biological system is an important phenomenon. The parameters derived from these equations have been interpreted in terms of solute-solute and solute-solvent interactions.
Keywords: Apparent molar volume, B-coefficient, density, Sodium Salicylate, viscosity,
—————————— ——————————
1. INTRODUCTION
Drugs of the analgesics, antipyretics, and anti- inflammatory class include a heterogeneous group of compounds. Many of these agents affect pain, fever and inflammation and are referred to as the non-steroidal anti- inflammatory drugs (NSAIDs).
The principle mechanism of action for all NSAIDs appears to be inhibition of prostaglandin synthesis by blocking the activity of the precursor enzyme, cyclooxygenase (COX). Their actions on the prostaglandins likely account for many of the side effects of the NSAIDs. Although, in general, there is little difference between the efficacies of different NSAIDs, some patients may respond to one agent better than another. This is difficult to predict and often necessitates trial and error to find the most suitable drug.
The discovery that NSAIDs inhibit prostaglandin biosynthesis was made by John Vane and coworkers1 in 1970. Tissue injury activates an enhanced conversion of arachidonic acid to prostaglandins via the COX pathway,
————————————————
Arun B. Nikumbh
P. G. Department of Chemistry, S. S. G. M. College, Kopargaon-423601. (M. S.) India aob.nikumbh@gmail.com
Ravindra C. Bhujbal
Department of Chemistry, Hon. B. J. College, Ale, Pune- 412411. (M. S.) India
rcbhujbal@gmail.com
Affiliated to University of Pune, Maharashtra State, India-411007
this is so named because COX enzyme catalyzes the conversion. Because some prostaglandins amplify pain
signals, inhibition of COX results in analgesia. NSAIDs have good analgesic efficacy, but less than that of opioids, a relatively rapid onset; well known adverse effects, including potentially fatal gastrointestinal bleeding and disturbance of salt and water balance.
Bio-pharmaceutics is the study of factors influencing the extent and rate of absorption. The rate and amount of drug absorption depends on biological and physicochemical factors. During their way to site of action, drug molecules have to cross one or more membranous barrier, which are lipoidal in nature and have different size of pores.
Physicochemical factors include lipid solubility, salt complexation, dissolution rate, Viscosity and drug stability in GIT. Lipid soluble drugs more unionized and easily absorbed Na and K salts of weak acid have higher absorption rate than acids.
All the drugs in any solid dosage form or suspension
when administered will first change into drug solution in
body fluids. So, dissolution rate is important factor affecting
the rate of absorption. When a drug is more rapidly or
completely absorbed from solution, it is very likely that its
absorption will be dissolution limited.
Viscosity limits the dissolution rate and there by affect
the rapid absorption.
Eg. Aqueous solution of Na-Salicylate showed its rapid
appearance in plasma while the same drug in suspension
form failed to reach the target as quickly as with aqueous
solution2.
The parameters like apparent molar volume, density,
viscosity ‘A’ and ‘B’ coefficient and Jones-Dole parameters
are useful to focus the solute-solvent interactions and to
understand different biochemical reactions at 310.15 K i.e. at
body temperature. It also enables to enrich the data at
various compositions. The results are interpreted in terms of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 139
ISSN 2229-5518
solute–solvent and solute–solute interactions in these systems.
2. EXPERIMENTAL
2.1 Materials:
Sodium salicylate of high purity was obtained from
Фv = ф0 + Sv.C½ (2)
Where ф0 is the limiting apparent molar volume and Sv a
semi-empirical parameter which depends on the nature of
solvent, solute and temperature.
The viscosity results for the aqueous solutions of drugs
were plotted in accordance with Jones-Dole equation8
Loba Chemie Pvt Limited, Mumbai, recrystallized and then used. Deionized water with a specific conductance of < 10-6
S.cm-1 was used for the preparation of solutions at room

C½ = A + BC
(3)
temperature in a molarity range (8.0×10-3 to 1.99×10-2)
mol.L-1. The precision of balance used was ±1×10-5g.
2.2 Density measurements:
The bicapillary pycnometer was calibrated by measuring
the densities of triple distilled water. The densities of
distilled organic liquids like acetone, alcohol, benzene,
carbon tetra chloride, aniline, and nitrobenzene were
evaluated with respect to density of water. The density was
measured with an uncertainty of ± 1.48 ×10-4g.cm-3.
2.3 Viscosity measurements:
The solution viscosities were measured with an uncertainty of ± 2.4×10-4 mPa.s by using Ubbelohde viscometer. The viscosity measurements were performed at
298.15, 303.15, 308.15, 310.15 and 310.15K. The temperature of thermostat is maintained to desired temperature, by using demerstat with an accuracy of ±0.1 K. The flow time will be measured at the accuracy of ±0.01 s.
The different compositions (0.0199M to 0.0080M) of solutions of Sodium salicylate were prepared in NS, DNS, RL and pure water. The viscosities were measured at different temperatures for seven different concentrations. The solvent system compositions used were as under,
• NS = (0.9 g NaCl) / 100 ml D.W.
• DNS = (5% Dextrose + 0.9 g NaCl) / 100 ml D.W.
• RL = (0.320 g Sodium Lactate + 0.600 g NaCl +
0.040 g KCl + 0.035 g CaCl2 .2H2 O) / 100 ml D.W.
• Distilled Water.
3. DATA EVALUATION
The apparent molar volumes, фv, were obtained from the
density results using the following equation 3-6
Where ηr = (η/ηo ) and η, ηo are viscosities of the solution
and solvent respectively, C is the molar concentration. The
linear plots for (ηr -1)/C½ versus C½ were obtained for the sodium salicylate. The B-coefficients were obtained from
the linear plots using the least-square fitting method. The A- coefficient reflects solute-solute interaction9 and the B- coefficient reflect the solute-solvent interactions.
4. RESULTS AND DISCUSSION
The values of the densities (ρ) and apparent molar volumes (фv ) of sodium salicylate solution in NS, DNS, RL and pure water at 298.15, 303.15, 308.15, 310.15 and 310.15K temperature are shown in Table 1 and 2. In all sets the densities of solutions increases with increase in concentration of solution. The фv values decreases as drug concentration increases except in pure water system.
Fig 1 shows the linear plots of фv vs C½ for sodium salicylate solutions in the NS solvent systems at different
temperatures. Similar plots were obtained in DNS, RL and pure water solvent systems. Masson’s parameters Фv0 and Sv were obtained from linear plots and are reported in table
3. The values of Фv0 obtained are positive for all the systems studied furnish important information regarding the drug hydrophobicity, hydration properties and solute- solvent interactions.
The Sv values are negative in NS, DNS and RL but in water system it is positive. The negative values of Sv for 0.08M NaCl as additive (-24443.8) were reported by Abdo Taher et. al10. Studies conducted on variety of compounds showed that negative values were associated with hydrophobic solutes11. The positive value indicates the presence of solute-solute interaction. The negative values of Sv for some solutes characterized by their water-structure promotion effects where the hydrophobic effect become
Φ𝑉 =


1000(𝜌𝜊 −𝜌) + ℳ
∁𝜌𝜊 𝜌
(1)
dominant compared with hydrophilic effect, therefore the
solvation around ionic moiety diminishes12.
The values of the viscosity and relative viscosities
where M, C, ρ and ρ0 are the molar mass of the Sodium salicylate, concentration (mol.L-1), and the densities of the
solution and the solvent, respectively.
The apparent molar volumes (фv ) were plotted against the square root of concentration (C½) in accordance with the Masson’s equation 7
of sodium salicylate in NS, DNS, RL and pure distilled water at 298.15, 303.15, 308.15, 310.15 and 310.15K are given in Table 4 and 5. In all sets the viscosities of solutions increases with increase in concentration of solution. Fig 2 shows the linear variation of (ηr -1)/C½ against square
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 140
ISSN 2229-5518
TABLE 1: DENSITIES AND APPARENT MOLAR VOLUMES OF SODIUM SALICYLATE SOLUTION IN NS AND
DNS AT DIFFERENT TEMPERATURES.
Molar Conc. of Sodium salicylate (C) mol/dm3 | Temperatures |
Molar Conc. of Sodium salicylate (C) mol/dm3 | 298.15K | 303.15K | 308.15K | 310.15K | 313.15K |
NS |
Densities (ρ), (g.cm-3) |
0.0080 | 1.00065 | 0.99915 | 0.99751 | 0.99677 | 0.99566 |
0.0100 | 1.00135 | 0.99984 | 0.99820 | 0.99739 | 0.99633 |
0.0120 | 1.00218 | 1.00062 | 0.99892 | 0.99816 | 0.99712 |
0.0140 | 1.00274 | 1.00125 | 0.99953 | 0.99887 | 0.99778 |
0.0160 | 1.00338 | 1.00185 | 1.00015 | 0.99943 | 0.99825 |
0.0180 | 1.00404 | 1.00254 | 1.00090 | 1.00016 | 0.99897 |
0.0199 | 1.00475 | 1.00325 | 1.00154 | 1.00082 | 0.99965 |
Apparent molar volumes (Ф v ) (cm3.mol-1) |
0.0080 | 509.59 | 520.29 | 511.18 | 502.85 | 493.43 |
0.0100 | 369.82 | 379.33 | 317.98 | 372.28 | 359.70 |
0.0120 | 265.85 | 277.87 | 276.68 | 272.73 | 260.54 |
0.0140 | 210.79 | 216.09 | 216.46 | 205.91 | 199.01 |
0.0160 | 164.53 | 171.62 | 170.67 | 165.18 | 164.75 |
0.0180 | 127.44 | 132.05 | 127.84 | 128.05 | 124.20 |
0.0199 | 94.947 | 99.094 | 98.762 | 94.317 | 93.423 |
DNS |
Densities (ρ), (g.cm-3) |
0.0080 | 1.00719 | 1.00578 | 1.00420 | 1.00351 | 1.00254 |
0.0100 | 1.00935 | 1.00824 | 1.00665 | 1.00602 | 1.00506 |
0.0120 | 1.01164 | 1.01019 | 1.00889 | 1.00854 | 1.00758 |
0.0140 | 1.01395 | 1.01228 | 1.01139 | 1.01100 | 1.01008 |
0.0160 | 1.01609 | 1.01443 | 1.01355 | 1.01345 | 1.01253 |
0.0180 | 1.01811 | 1.01672 | 1.01574 | 1.01564 | 1.01496 |
0.0199 | 1.02021 | 1.01875 | 1.01778 | 1.01762 | 1.01711 |
Apparent molar volumes (Ф v ) (cm3.mol-1) |
0.0080 | 2259.9 | 2231.8 | 2214.8 | 2207.9 | 2171.4 |
0.0100 | 1628.3 | 1576.2 | 1563.3 | 1551.7 | 1521.3 |
0.0120 | 1196.7 | 1180.7 | 1146.0 | 1113.4 | 1087.8 |
0.0140 | 887.00 | 888.45 | 829.81 | 804.52 | 779.60 |
0.0160 | 665.10 | 665.58 | 613.46 | 573.49 | 551.52 |
0.0180 | 499.02 | 484.62 | 443.56 | 407.59 | 375.21 |
0.0199 | 363.28 | 353.56 | 315.78 | 286.46 | 248.36 |
Table 2: Densities and apparent molar volumes of Sodium Salicylate solution in RL and pure water at different temperatures.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 141
ISSN 2229-5518
Molar Conc. of Sodium salicylate (C) mol/dm3 | Temperatures |
Molar Conc. of Sodium salicylate (C) mol/dm3 | 298.15K | 303.15K | 308.15K | 310.15K | 313.15K |
RL |
Densities (ρ), (g.cm-3) |
0.0080 | 0.99996 | 0.99861 | 0.99692 | 0.99605 | 0.99494 |
0.0100 | 1.00062 | 0.99928 | 0.99758 | 0.99665 | 0.99554 |
0.0120 | 1.00127 | 0.99994 | 0.99819 | 0.99724 | 0.99618 |
0.0140 | 1.00190 | 1.00056 | 0.99882 | 0.99788 | 0.99672 |
0.0160 | 1.00245 | 1.00115 | 0.99943 | 0.99845 | 0.99732 |
0.0180 | 1.00300 | 1.00175 | 1.00007 | 0.99894 | 0.99786 |
0.0199 | 1.00359 | 1.00231 | 1.00053 | 0.99951 | 0.99832 |
Apparent molar volumes (Ф v ) (cm3.mol-1) |
0.0080 | 570.74 | 581.44 | 579.94 | 586.67 | 586.07 |
0.0100 | 422.72 | 430.24 | 429.98 | 441.34 | 440.83 |
0.0120 | 324.87 | 330.27 | 334.17 | 345.29 | 340.67 |
0.0140 | 256.41 | 261.72 | 264.31 | 273.10 | 276.27 |
0.0160 | 210.04 | 212.18 | 213.16 | 223.35 | 224.22 |
0.0180 | 173.98 | 173.09 | 171.72 | 189.09 | 187.08 |
0.0199 | 143.05 | 143.73 | 147.50 | 157.67 | 161.37 |
Pure water |
Densities (ρ), (g.cm-3) |
0.0080 | 0.99775 | 0.99634 | 0.99464 | 0.99387 | 0.99277 |
0.0100 | 0.99792 | 0.99648 | 0.99475 | 0.99393 | 0.99285 |
0.0120 | 0.99807 | 0.99662 | 0.99487 | 0.99401 | 0.99294 |
0.0140 | 0.99823 | 0.99677 | 0.99498 | 0.99411 | 0.99301 |
0.0160 | 0.99839 | 0.99690 | 0.99509 | 0.99419 | 0.99308 |
0.0180 | 0.99852 | 0.99703 | 0.99520 | 0.99428 | 0.99315 |
0.0199 | 0.99866 | 0.99716 | 0.99530 | 0.99438 | 0.99325 |
Apparent molar volumes (Ф v ) (cm3.mol-1) |
0.0080 | 71.562 | 80.446 | 89.38 | 93.222 | 93.325 |
0.0100 | 72.314 | 82.455 | 92.649 | 100.77 | 98.868 |
0.0120 | 74.487 | 83.794 | 93.991 | 104.13 | 101.72 |
0.0140 | 75.323 | 84.033 | 95.667 | 105.09 | 105.20 |
0.0160 | 75.950 | 85.467 | 96.925 | 107.06 | 107.81 |
0.0180 | 78.109 | 86.583 | 97.903 | 108.04 | 109.84 |
0.0199 | 78.927 | 87.108 | 98.877 | 108.06 | 109.70 |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 142
ISSN 2229-5518
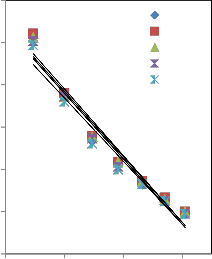
600
500
400
298.15
303.15
308.15
310.15
313.15
Table 3: Фv0 (cm3.mol-1) and S v (cm3 .mol-3/2 .L1/2) of sodium salicylate solutions in NS, DNS, RL and pure water at different temperatures.
300
200
100
0
0.08 0.1 0.12 0.14
Root of Conc. (mol½.dm-3/2)
Fig 1: Plot of Фv (cm3.mol-1) Versus C½ (mol1/2.dm-3/2) for sodium salicylate solution in NS at T= 298.15 to 313.15K.
root of concentration at different temperatures for different solvent systems performed. ‘A’ is constant independent of concentration and ‘B’ is Jones-Dole coefficient represents measure of order and disorder introduced by solute into
the solution. Positive ‘B’- coefficient shows strong alignment of solvent towards solute and is related to the effect of the ions on the structure of water 13. The Jones-Dole parameters are given in Table 6. The positive values of ‘B’ at all temperatures indicate water structuring14. Increase in
‘B’ value shows increase in kosmotropicity and B values follows the trend
BDNS > B RL > BNS > B D.W.
5. CONCLUSIONS
In the present report, from densitometry and viscometric
study of aqueous solutions of sodium salicylate and in
presence of additives as NaCl, KCl, Dextrose, Sodium
lactate etc. at different temperatures are systematically
presented. It has been observed that there exist strong
solute–solvent interactions in these systems, which
increases with increase in pain killer concentration.
The values of фv0 are positive suggest strong ion-solvent interactions. The Sv values are negative (except in pure
water system) hydrophobicity is dominant over hydrophilic effect. The positive values of Jones-Dole coefficient ‘B’ indicates structure promoting tendency and strong interactions between solute and solvent. In aqueous solutions the values of partial molar volume are positive and decrease with the extent of hydrogen bonding. Lower the partial molar volume value, stronger is the hydrogen bond. Positive values of ‘B’ suggesting strongly hydrated solute indicating structure promoting tendency i.e. kosmotropes (structure makers). The Jones-Dole and Masson’s equations are found to obey the sodium salicylate solutions in NS, DNS, RL and pure water system.

Table 4: Viscosities and relative viscosities of sodium salicylate solution in NS and DNS at different temperatures.
Molar Conc. Of Temperatures
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 143
ISSN 2229-5518
Sodium salicylate
(C) mol/dm3 298.15K 303.15K 308.15K 310.15K 313.15K
NS
Viscosities (η) (Nm-3.s)
0.0080 | 0.90686 | 0.81175 | 0.73245 | 0.70603 | 0.66562 |
0.0100 | 0.91007 | 0.81401 | 0.73437 | 0.70805 | 0.66673 |
0.0120 | 0.91274 | 0.81612 | 0.73639 | 0.71017 | 0.66923 |
0.0140 | 0.91478 | 0.81846 | 0.73887 | 0.71242 | 0.6708 |
0.0160 | 0.91698 | 0.82061 | 0.74071 | 0.71412 | 0.67273 |
0.0180 | 0.91949 | 0.82283 | 0.74337 | 0.71628 | 0.67389 |
0.0199 | 0.92154 | 0.82477 | 0.74535 | 0.71785 | 0.67621 |
Relative viscosities (ηr ) |
0.0080 | 0.99633 | 0.98668 | 0.99159 | 0.99428 | 0.99771 |
0.0100 | 0.99986 | 0.98942 | 0.99419 | 0.99713 | 0.99937 |
0.0120 | 1.00279 | 0.99199 | 0.99693 | 1.00011 | 1.00312 |
0.0140 | 1.00503 | 0.99483 | 1.00028 | 1.00328 | 1.00547 |
0.0160 | 1.00745 | 0.99745 | 1.00277 | 1.00567 | 1.00836 |
0.0180 | 1.01021 | 1.00015 | 1.00638 | 1.00872 | 1.01010 |
0.0199 | 1.01246 | 1.00250 | 1.00906 | 1.01093 | 1.01358 |
| | DNS | | | |
Viscosities (η) (Nm-3.s) |
0.0080 | 0.94989 | 0.85084 | 0.76948 | 0.73896 | 0.69586 |
0.0100 | 0.96348 | 0.86422 | 0.78118 | 0.74966 | 0.70425 |
0.0120 | 0.98026 | 0.87911 | 0.79194 | 0.76204 | 0.71486 |
0.0140 | 0.99497 | 0.89507 | 0.80492 | 0.77262 | 0.72219 |
0.0160 | 1.00865 | 0.90251 | 0.81538 | 0.78341 | 0.73222 |
0.0180 | 1.02055 | 0.91957 | 0.82429 | 0.79229 | 0.74111 |
0.0199 | 1.03375 | 0.92632 | 0.83287 | 0.79863 | 0.75004 |
Relative viscosities (ηr ) |
0.0080 | 0.91378 | 0.91477 | 0.91813 | 0.91784 | 0.92075 |
0.0100 | 0.92685 | 0.92916 | 0.93210 | 0.93113 | 0.93186 |
0.0120 | 0.94299 | 0.94517 | 0.94493 | 0.94650 | 0.94589 |
0.0140 | 0.95714 | 0.96233 | 0.96042 | 0.95964 | 0.95559 |
0.0160 | 0.97030 | 0.97033 | 0.97290 | 0.97305 | 0.96886 |
0.0180 | 0.98175 | 0.98867 | 0.98353 | 0.98408 | 0.98063 |
0.0199 | 0.99445 | 0.99593 | 0.99377 | 0.99195 | 0.99244 |
Table 5: Viscosities of sodium salicylate solution in RL and pure water at different temperatures.
Molar Conc. of Sodium salicylate | Temperatures |
Molar Conc. of Sodium salicylate | 298.15K | 303.15K | 308.15K | 310.15K | 313.15K |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 144
ISSN 2229-5518
(C) mol/dm3
RL
Viscosities (η) (Nm-3.s)
0.0080 | 0.91279 | 0.81792 | 0.73795 | 0.70964 | 0.66921 |
0.0100 | 0.91656 | 0.82095 | 0.74008 | 0.71125 | 0.67145 |
0.0120 | 0.91985 | 0.82362 | 0.74235 | 0.71407 | 0.67339 |
0.0140 | 0.92269 | 0.82569 | 0.74541 | 0.71674 | 0.67545 |
0.0160 | 0.92587 | 0.82953 | 0.74921 | 0.71958 | 0.67749 |
0.0180 | 0.92965 | 0.83266 | 0.7513 | 0.72139 | 0.68021 |
0.0199 | 0.93256 | 0.83511 | 0.75311 | 0.72332 | 0.68342 |
Relative viscosities (ηr ) |
0.0080 | 1.00908 | 0.99732 | 0.98102 | 0.97354 | 0.98174 |
0.0100 | 1.01324 | 1.00101 | 0.98385 | 0.97574 | 0.98502 |
0.0120 | 1.01688 | 1.00427 | 0.98687 | 0.97961 | 0.98787 |
0.0140 | 1.02002 | 1.00679 | 0.99093 | 0.98328 | 0.99089 |
0.0160 | 1.02354 | 1.01147 | 0.99598 | 0.98717 | 0.99388 |
0.0180 | 1.02771 | 1.01529 | 0.99876 | 0.98966 | 0.99787 |
0.0199 | 1.03093 | 1.01828 | 1.00117 | 0.99230 | 1.00258 |
| | Pure water | | | |
Viscosities (η) (Nm-3.s) |
0.0080 | 0.90185 | 0.80945 | 0.73155 | 0.70289 | 0.66215 |
0.0100 | 0.90331 | 0.81106 | 0.73298 | 0.70433 | 0.66308 |
0.0120 | 0.90448 | 0.81219 | 0.7347 | 0.70538 | 0.66398 |
0.0140 | 0.90596 | 0.81369 | 0.73575 | 0.7066 | 0.66479 |
0.0160 | 0.90718 | 0.81536 | 0.73726 | 0.70818 | 0.66603 |
0.0180 | 0.90909 | 0.81668 | 0.73863 | 0.70966 | 0.66701 |
0.0199 | 0.91073 | 0.81795 | 0.73988 | 0.71098 | 0.66817 |
Relative viscosities (ηr ) |
0.0080 | 1.00915 | 1.01903 | 1.01251 | 1.01182 | 1.00937 |
0.0100 | 1.01079 | 1.01291 | 1.01449 | 1.01389 | 1.01079 |
0.0120 | 1.01210 | 1.01432 | 1.01687 | 1.01540 | 1.01216 |
0.0140 | 1.01375 | 1.01620 | 1.01832 | 1.01716 | 1.01340 |
0.0160 | 1.01512 | 1.01828 | 1.02041 | 1.01943 | 1.01529 |
0.0180 | 1.01725 | 1.01993 | 1.02231 | 1.02156 | 1.01678 |
0.0199 | 1.01909 | 1.02152 | 1.02404 | 1.02346 | 1.01855 |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 145
ISSN 2229-5518
0.15
0.1
0.05
0
-0.05
-0.1
-0.15
-0.2
298.15
303.15
308.15
310.15
313.15
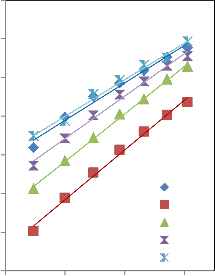
0.08 0.1 0.12 0.14
C½
REFERENCES:
[1] ANALGESICS, ANTIPYRETIC, AND B Raffa, PhD, Remington; The Science and
Practice of Pharmacy,21st edition, vol-II, LippincottWilliams&Wilkins, Indian edition, 21st edition, 2005, Ch 83, p 1524.
Singh, Academa publishers, 2004, 1st
Chapter 1.2, Page No.8-10.
[3] Nikam, P.S., Ansari, H.R. and Hasan, M.,
(1999). J. Indian Chem. Soc. 76: 344-346.
[4] Arun B. Nikumbh*a, Ganesh K. Kulkarnia
and Ravindra C. Bhujbalb, “Study of flow
and interaction parameters of Aspirin in
NS, DNS, RL, and pure water system at
298.15K, 303.15K, 308.15K, 310.15K and
313.15K”. International Journal of
Innovative Research in Science,
Fig 2: Plot of (ηr -1)/ C½ vs C½ for sodium salicylate solutions in NS solvent system at different temperatures.
Table 6: Parameters of Jones-Dole equation of sodium salicylate in NS, DNS, RL and pure water.
Engineering and Technology, Volume 2, Issue 9, September 2013, ISSN: 2319-8753.
[5] Md. Monimul Huque, A.N.M. Hamidul Kabir, Md. Nurul Huda, Shaila Kabir Bangladesh Pharmaceutical Journal; Vol.
13, No. 2, July 2010.
[6] Muhammad Javed Iqbal, Mansoora
Ahmed Chaudhry, Thermodynamic study
of three
pharmacologically significant drugs:
Density, viscosity, and refractive index
measurements at different temperatures. J.
Chem. Thermodynamics 41 (2009) 221–
226.
[7] S. Chauhan, V.K. Syal, M.S. Chauhan,
Poonam Sharma, Viscosity studies of
some narcotic– analgesic drugs in
aqueous–alcoholic mixtures at 25 °C, J.
Mol. Liqs.136 (2007) 161-164.
[8] G. Jones, M. Dole, The viscosity of
aqueous solutions of strong electrolytes with special reference to barium chloride J. Am. Chem. Soc. 51(1929) 2950-2964.
[9] H Falkenhagen, M Dole, Viscosity of electrolyte solutions and its significance to the Debye theory. Zeitschrift Fur Physik
30 (1929) 611-616.
[10] Abdo Taher, Mohammad Mohsin and
Mazahar Farooqui, “Density, viscosity and conductance measurement of
pyrazinamide in aqueous solution in presence and absence of additives at
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 146
ISSN 2229-5518
308.15K”, J. Indian Chem. Soc.Vol 88, July
2011, pp 959-962.
[11] M. J.Iqbal and Q.M. Malik, “Partial molar
volume of paracetamol in water, 0.1 M
HCl and 0.154 M NaCl atT = (298.15,
303.15, 308.15 and 310.15) K and at
101.325kPa”, J. Chem. Thermodynamics,
37 (2005) 1347-1350.
[12] Daniel R.Delgado, Alvaro F, Jimenez-
Kairuz, Ruben H Manzo, Edgar F Vargas,
Fleming Martinez, Rev.Colomb.
Cienc.Quim. Farm., “Apparent molar
volumes of the anesthetic drugs procaine-
HCl and lidocaine-HCl in water at
temperatures 278.15 to 313.15K.”Vol 39 (1)
(2010) 57-67.
[13] R. A. Robinson, R. H. Stokes, Electrolytic
solutions, Butterworth scientific
publications, London,1959.
[14] B.Haribar,N.T.Southall, V. Vlachy, K.A.
Dill, J. Am. Chem. Soc. 124(2002)12302.
IJSER © 2014 http://www.ijser.org



