International Journal of Scientific & Engineering Research Volume 2, Issue 8, August-2011 1
ISSN 2229-5518
Antimicrobial Silver Nanomaterials
Synthesized by HVPCG Technique
Wilfred V. Espulgar, Gil Nonato C. Santos
Abstract—Triangular silver nanoplates, of different orientations, and other nanostructures were successfully synthesized using the Horizontal Vapor Phase Crystal (HVPC) Growth technique for antimicrobial purposes in this study. This finding demonstrates HVPC as an alternative and simple technique to synthesize ordered silver nanomaterials for antimicrobial study.
Keyword —alternative method, antimicrobial, evaporation-condensation process, framework building, top-down technique,
silver nanomaterials, solid route
.
1 INTRODUCTION
—————————— ——————————
NTEREST has arisen in the manufacture and the cha- racterization of silver nanomaterials because of their unusually enhanced physicochemical properties and biological activities compared to the bulk parent mate- rials or macro scaled counterparts. These properties provide the wide potential use of silver for numerous applications like antimicrobial applications in medical devices and supplies and in various consumer products [1], [2], [3], [4], utilization of fluorescence and surface plasmon resonance (SPR) for sensing applications [5], [6],
[7], [8], and span in electronic applications [9], [10], [11].
Among the properties and applications of silver na-
nomaterials, its antimicrobial property earns the greatest
interest among the existing studies because of its simplic-
ity and great importance. The search for a better and
stronger antimicrobial agent is unending because of the
microorganisms’ continuous adaptation to develop re- sistance to the manufactured drugs.
Bulk silver is known for its antimicrobial properties
and has been used for several years in the medical field
for antimicrobial applications. Silver has even shown to
prevent HIV binding to host cells [12]. In fact, colloidal
silver or the bulk form of silver was used as an antibiotic
before the discovery of penicillin. In addition, silver had
been used in water and air filtration to eliminate micro-
organisms [13], [14], [15]. This antimicrobial property of
bulk silver is expected to be carried over and, perhaps,
enhanced in silver nanomaterials. However, the hypothe-
sized mechanisms that govern the fate and transport of
bulk materials may not be directly applied to materials at
the nanoscale and thus, remains to be understood. Sever-
al studies proposed that silver nanoparticles may attach
————————————————
Wilfred V. Espulgar, Master of Science in Physics, Lecturer, De La Salle
University-Manila, Philippines. E-mail: espulgarw@dlsu.edu.ph
Gil Nonato C. Santos, Doctor of Philosophy in Materials Science, Profes-
sor, De La Salle University-Manila, Philippines. E-mail:
santosg@dlsu.edu.ph
to the surface of the cell membrane, disturbing the per- meability and the respiration functions of the cell [16]. It is also possible that the silver nanoparticles do not only interact with the surface of membrane but can also pene- trate inside the bacteria [17]. Also, the shape of silver nanomaterials may interfere with their antimicrobial ef- fect. Triangular silver nanomaterials displayed greater biocidal action against E. coli than rod or spherical na- nomaterials. [18]. Still, the question remains on when and how silver nanomaterials can be a microbicidal or a mi- crobistatic and the effects of different nanostructures to this behavior.

Different synthesizing techniques have been em- ployed to successfully grow silver nanomaterials of dif- ferent structures. Majority of the techniques employed suggested that the bottom-up technique is predominant- ly used in the synthesis of silver nanomaterials relative to the top-down technique [19]. However, this majority posed environmental issues associated with the manu- facture while taking into account its antibacterial effect [19]. This is due to the production of other chemicals aside from the material of interest, where disposal is a major concern.
This study presents the use of the Horizontal Vapor
Phase Crystal (HVPC) Growth Techique as an alternative
technique in synthesizing silver nanomaterials for anti-
microbial purposes. The major advantages of this tech-
nique, employing the evaporation-condensation process,

compared to liquid phase routes are higher purity and
good thermal stability [20]. The synthesized and charac-
terized silver of different nanostructures grown in a sim-
ple technique, hopefully, will be helpful as a contribution
for building a framework in understanding its antimi-
crobial property by providing researchers the desired
silver nanostructures.
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 8 , August -2011 2
ISSN 2229-5518
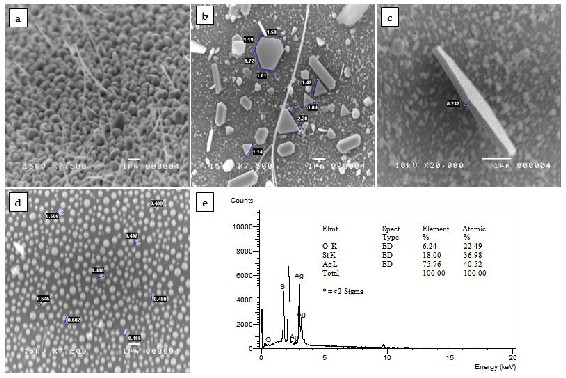
Fig.1. SEM images of the synthesized silver nanomaterials: (a) nanoparticles and nanowries, (b) triangular and hexagonal nanoplates in different sizes, (c) nanoplate with 232-nm thickness, and (d) spherical nanoparticles with average size of 500 nm in diameter; and (e) EDX spectrum of a triangular silver nanoplate.
2 EXPERIMENTAL SECTION
2.1 Synthesis and Characterization of Ag
Nanomaterials
The starting material for the synthesis was thirty-five (35) mg of 99.99 % pure silver powder ordered from Aldrich Corporation. Silver nanomaterials were synthesized us- ing the Horizontal Vapor Phase Crystal (HVPC) Growth Technique; a procedure that is similar to a previous Mas- ter’s Thesis *21+. Briefly, a vacuumed (~ 10-6 Torr) and fully sealed amorphous silica tube containing the silver powder was inserted halfway through a Thermolyne furnace. The furnace was set at a certain growth tempera- ture (800 °C, 900 °C, 1000 °C, or 1100 °C) and at a certain growth time (4 hours, 6 hours, or 8 hours). After cooling to room temperature, the products were collected for characterization using a scanning electron microscope (JEOL 5310) and energy dispersive x-ray analysis (Ox- ford with Link Isis). Parametric analyses on the growth time, the growth temperature, and the zone of deposition were done to determine the optimum parameters for the desired structures of silver.
2.2 Antimicrobial Test of the Grown Ag
Nanomaterials
Pour-plate technique was employed to confirm that the antimicrobial property of bulk silver is carried over or perhaps enhanced in the grown silver nanomaterials. A
105 CFU/mL of E coli was prepared through serial dilu-
tion of the original 108 CFU/mL bacterial solution. Four sealed amorphous silica tubes were prepared for this test: tube a - a tube that doesn’t contain silver but was placed inside the furnace, tube b - a tube that contains 35 mg of silver powder but was not placed inside the fur- nace, and tubes c and d - two tubes that contain silver nanomaterials (mostly triangular nanoplates). The ends of the tubes (the ones exposed outside the furnace) were cracked and served as the entry points of the bacterial solution. Five milliliters of the bacterial solution was poured into the silica tubes and was then shaken for 30 minutes using an orbital shaker. One mL of the bacterial solution from each silica tube is poured in separate ste- rile petri dishes to which is then poured 9 mL of sterile and cold (at 45 °C) nutrient agar medium. The contents were thoroughly mixed and allowed to solidify. After incubating at 35 °C for 24 hours, the plates were then compared.
3 RESULTS AND DISCUSSION
Fig. 1 presents some of the SEM images of the silver na- nostructures synthesized in this technique. Structures such as nanowires, nanorods, triangular and hexagonal nanoplates, and nanoparticles were successfully synthe- sized. Optimum size and number of nanoplates, whether triangular or hexagonal, were best grown at a low growth temperature of 800°C and a short growth time (4 hours and/or 6 hours). The desired size of spherical na-
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 8 , August -2011 3
ISSN 2229-5518
noparticles can be achieved by increasing the growth time regardless of the growth temperature. Optimum thickness and length of the nanowires and nanorods are best grown at a high growth temperature (1100°C) and at a short growth time (4 hours). Such result is in coherence to the vapor-deposition process [22]. Deposition of par- ticles was affected by the growth temperature. The silver powder in vacuum is believed to melt at 800°C. At 800°C and 900 °C, vaporization is believed to be slow that pro- moted the growth of 2-dimensional nanostructures. At
1000 °C and 1100°C, vaporization is believed to be fast that promoted the growth of one-dimensional nanostruc- tures.
Synthesized triangular silver nanoplates were sub- jected for antimicrobial test as it has the greatest biocidal action to E. coli [18]. The energy-dispersive x-ray analysis (Fig. 1e) of a sample triangular nanoplate provides evi- dence that the product grown was indeed silver. The Si and O peaks are attributed to the amorphous silica tube substrate.
Fig. 2 presents the result of the antimicrobial test us- ing the pour plate technique. It can be seen from the pic- tures that the number of colonies in plates c and d are lesser, compared to plates a and b. Comparing plates 1 and 2 does not show any significant difference. This indi- cated that the 35 mg of silver powder was not enough to kill E. coli in a 105 CFU/mL bacterial solution within 30 minutes.
However in plates c and d, there was a distinct dif- ference from plate b. Plates c and d had a lesser number of grown colonies as compared to plate b. This indicated that the antimicrobial property of bulk silver was not only carried over but was enhanced on the grown silver nanomaterials counterpart. Since, the most number of triangular nanoplates were synthesized and that they have high {111} active facets, such enhancement was due to the increase in surface contact of the silver atoms to the bacteria [18] and their high surface to volume ratio.
4 CONCLUSION
This study demonstrated the potential of the Horizontal Vapor Phase Crystal (HVPC) Growth Technique in syn- thesizing silver nanomaterials for antimicrobial study and purposes. The grown silver nanomaterials exhibited enhanced antimicrobial property that is consistent and comparable with other studies. The synthesis does not require of any templates or surfactants since the process is in solid route and thus eliminating the factor of un- wanted wastes and its disposal. Thus, this finding pro- motes the HVPC technique as an alternative and simple technique for the said purpose.
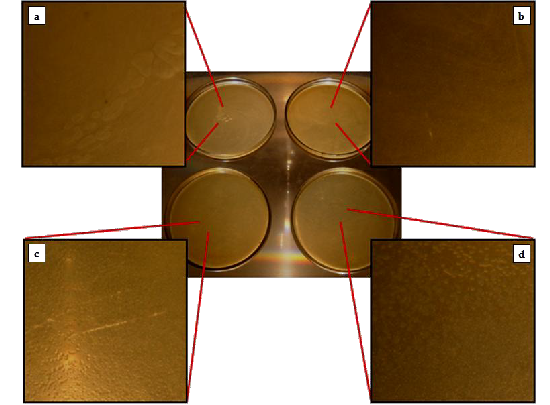
Fig.2. E. coli colonies grown when 105 CFU/mL of bacterial solution was exposed to (a) a plane amorphous silica tube, (b) an amorphous silica tube with silver powder, and (c-d) two amorphous silica tubes with grown silver nanomaterials (mostly triangular plates)
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 8 , August -2011 4
ISSN 2229-5518
ACKNOWLEDGMENT
This work was supported in part by a grant from the Department of Science and Technology-Science Educa- tion Institute, Philippines.
REFERENCES
[1] Polizzi, and M. Meneghetti, ‚Free silver nanoparticles synthe- sized by laser ablation in organic solvents and their easy func- tionalization,‛ Langmuir, vol. 23, pp. 6766–6770, 2007.
[2] S. Navaladian, B. Viswanathan, T.K. Varadarajan, and R.P.
Viswanath, ‚Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation,‛ Nanotechnolo- gy, vol.19, pp. 1–7, 2008.
[3] E.J. Fernandez, J. Garcıa-Barrasa, A. Laguna, J. Lopez-de- Luzuriaga, M. Monge, and C. Torres, ‚The preparation of highly active antimicrobial silver nanoparticles by an organo- metallic approach,‛ Nanotechnology, vol. 19, pp. 1–6, 2008.
[4] V. Thomas, M.M. Yallabu, B. Sreedhar, and S.K. Bajpai, ‚A ver- satile strategy to fabricate hydrogel–silver nanocomposites and investigation of their aAntimicrobial activity,‛ J Colloid Interface Sci, vol. 315, pp. 389–395, 2007.
[5] J.A. Jacob, H.S. Mahal, N. Biswas, T. Mukherjee, and S. Kapoor,
‚Role of phenol derivatives in the formation of silver nanopar-
ticles,‛ Langmuir, vol. 24, pp. 528–533, 2008.
[6] L. Hua, J. Chen, L. Ge, and S.N. Tan, ‚Silver nanoparticles as matrix for laser desorption/ionization mass spectrometry of peptides,‛ J Nanopart Res, vol. 9, pp.1133–1138, 2007.
[7] B.J. Wiley, Y. Chen, J.M. McLellan, Y. Xiong, Z. Li, D. Ginger, et al., ‚Synthesis and optical properties of silver nanobars and nanorice,‛ Nano Letters, vol. 7, pp. 1032–1036, 2007.
[8] S.T. Dubas and V. Pimpan, ‚Humic acid assisted synthesis of silver nanoparticles and its application to herbicide detection,‛ Mater Lett B, vol. 62, pp. 2661–2663, 2008.
[9] S. Navaladian, B. Viswanathan, T.K. Varadarajan, and R.P.
Viswanath, ‚Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation,‛ Nanotechnolo- gy, vol. 19, pp. 1–7, 2008.
[10] S.J. Kim, T.G. Kim, C.S. Ah, K. Kim, and D. Jang, ‚Photolysis dynamics of benzyl phenyl sulfide adsorbed on silver nano- particles,‛ J Phys Chem B, vol. 108, pp. 880–882, 2004.
[11] R.D. Deshmukh and R.J. Composto, ‚Surface segregation and formation of silver nanoparticles created in situ in poly(methyl methacrylate) films,‛ Chem Mater, vol. 19, pp. 745–754, 2007.
[12] N. Nino-Martınez, G.A. Martınez-Castanon, A. Aragon-Pina, F.
Martınez-Gutierrez, J.R. Martınez-Mendoza, and F. Ruiz,
‚Characterization of silver nanoparticles synthesized on tita-
nium dioxide fine particles,‛ Nanotechnology, vol. 19, pp. 1–8,
2008.
[13] W-L.Chou, D-G. Yu, and M-C.Yang, ‚The preparation and
characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment,‛ Polymers for Advance Technolo- gy, vol. 16, pp. 600-607, 2005.
[14] M. Jin, X. Zhang, S. Nishimoto, Z. Liu, D.A. Tryk, A.V. Emeline,
et al., ‚Light-stimulated composition conversion in TiO2-based
nanofibers,‛ Journal of Physical Chemistry C, vol. 111(2), pp. 658-
665, 2007.
[15] Q. Chen, L. Yue, F. Xie, M. Zhou, Y. Fu, Y. Zhang, et al., ‚Prefe- rential facet of nanocrystalline silver embedded in polyethy-
lene oxide nanocomposite and its antibiotic behaviors,‛ Journal of Physical Chemistry C, vol. 112(27), pp. 10004-10007, 2008.
[16] L. Kvitek, A. Panacek, J. Soukupova, M. Kolar, R. Vecerova, R.
Prucek, et al., ‚Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs),‛ Journal of Physical Chemistry C, vol. 112(15), pp. 5825-5834, 2008.
[17] J.R.Morones, J.L. Elechiguerra, A. Camacho, K. Holt, J. Kouri, J.T. Ramirez, et al., ‘The bactericidal effect of silver nanopar- ticles,” Nanotechnology, vol. 16, pp. 2346-2353, 2005.
[18] S. Pal, Y.K. Tak, and J.M. Song, ‚Does the antibacterial activity of silver nanoparticles depend on the shape of the nanopar- ticle? A study of the gram-negative bacterium Escherichia co- li,‛ Appl Environ Microbiol, vol. 73, pp. 1712–1720, 2007.
[19] T. Tolaymat, A. El Badawy, A. Genaidy, K. Scheckel, T. Luxton, and M. Suidan, An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applica- tions: a systematic review and critical appraisal of peer- reviewed scientific papers,‛ Science of the Total Environment, vol. 408, pp. 999-1006, 2010.
[20] K. Lehtinen, U. Backman, J. Jokiniemi, and M. Kulmala,
‚Three-body collisions as a particle formation mechanism in silver nanoparticle synthesis,‛ Journal of Colloid and Interface Science, vol. 274, pp. 526–530, 2004.
[21] G. Castillon, ‚Synthesis and characterization of Indium (III) Oxide nanomaterials grown via horizontal vapor phase crystal growth technique,‛ M.S. Physics thesis, De La Salle University- Manila, Philippines, 2009.
[22] G. Cao, Nanostructures & Nanomaterials: Synthesis, Properties & Applications, London: Imperial College Press, 2004.
IJSER © 2011
http://www.ijser.org



