International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 277
ISSN 2229-5518
Antihyperglycemic potential of urticol from Urtica dioica leaves using freshly isolated rat hepatocytes
*Charles K. Rono1, Geoffrey K. Maiyoh2 and Clare I. Muhanji3
1Department of Chemistry and Biochemistry, University of Eldoret, P.O Box 1125-30100, Eldoret-Kenya
2Department of Medical Biochemistry, School of Medicine, Moi University, P.O Box 3900-30100, Eldoret-Kenya
3Department of Chemical Sciences and Technology, Technical University of Kenya, P.O Box 58428-00200, Nairobi-Kenya

*corresponding author, ronock13@gmail.com
Abstract: The present study investigated the potential upregulation of glucose absorption in primary rat hepatocytes by compounds from Urtica dioica leaves (UD), isolated in our previous study. The isolates were then subjected to ex vivo assays using primary rat hepatocytes incubated under humidified 5 % CO2 incubator at 37 oC. It was found out that urticol at 100µM stimulated glucose uptake by 28.57 % and 11.45 % as compared to the untreated control and pioglitazone at 100 µM respectively (p≤0.05) while the terpenoids were found to be non-bioactive at both 50 µM
and 100 µM. This showed that urticol is stronger than pioglitazone in stimulation of glucose uptake. Therefore, the findings show urticol as a potent antihyperglycemic agent. This work may provide a scientific proof of the folklore antidiabetic activity of UD. Further work needs to be done to fully understand the exact mechanism of action of urticol in inducing glucose uptake. It is hypothesized that urticol may work by inducing Glut-4 expression on
hepatocytes but this remains to be determined.
Key words: ex vivo, urticol, hepatic glucose uptake, rat hepatocytes, antihyperglycemic, Urtica dioica, DCM extract
INTRODUCTION
Diabetes is the most common endocrine disorder affecting mankind all over the world, prevalence of which is increasing every day [1, 2]. It is a clinical syndrome characterized by hyperglycemia caused by a relative or absolute deficiency of insulin at the cellular level. Diabetes is associated with several structural and functional liver abnormalities that affect glycogen and lipid metabolism [3, 4]. Despite the availability of many synthetic drugs, a large number of diabetic patients seek herbal medication to relieve the symptoms of the disease [5, 6].
Urtica dioica (UD) in folk medicine of most cultures has emerged the best drug candidate as antidiabetic [7]. UD is a herbaceous perennial plant native to Europe, Asia, North America and Africa [3]. In Kenya, the plant is native to Rift Valley, Central and Western parts of Kenya. The nettle has a lot of medicinal value especially in improving certain diseases like diabetes [8, 9]. α-glucosidase inhibitory activity in UD extract was conducted to identify a prophylactic effect for diabetes [10]. The inhibitory
effect of this plant's extract and some common
antidiabetic drugs against the enzyme source (liver and small intestine) was reported [11]. Also, Daher et al., (2006) [12] reported that orally administered UD extract, improve the blood lipid profile. Moreover, hyperglycemia is responsible for the development of oxidative stress via glucose auto-oxidation and protein glycotion which is characterized by increased lipid peroxide production (malondialdehyde) and or decreased antioxidative defence [13, 14]. A further study conducted by Gulcin et al., (2004) [7] revealed vast medicinal value of the UD some of which include analgesic, antiulcer, antimicrobial and antioxidant activities of the nettle. The water extract of the nettle was observed to have a powerful antioxidant activity against various oxidative systems in vitro. The various antioxidant mechanisms of the extract may be attributed to strong hydrogen donating ability, metal chelating ability, and their effectiveness as scavengers of hydrogen peroxide, superoxide, and free radicals. In the study, phenolic compounds were suggested to be responsible for the antioxidant activity of the water extract of the nettle. Therefore,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 278
ISSN 2229-5518
the water extract of the nettle can be used as an accessible source of natural antioxidants and as a possible food supplement or in pharmaceutical industry [7]. Briefly, free radicals have been demonstrated to be a contributing factor in the tissue injury and modulation of pain in diabetics [15]. Further work on antidiabetic activity of UD [16] revealed that there is induction of insulin secretion by a component of UD extract in perfuses islets of Langerhans and its in vivo effects in STZ induced diabetic rats. Bnouham et al., (2003) [17] observed that aqueous extract of UD showed antihyperglycemic activity on STZ induced rats.
Although there are some other advancement in therapeutic techniques recently developed which include islets transplantation, there are a lot of problems challenging the technique. These include severe shortage of islets available for transplant and the need for patients/islets recipients to take
RESULTS AND DISCUSSIONS
The previous study on isolation and characterization of UD leaves DCM extract generated structures of 4 compounds; one of which compound
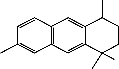
1, shown below, is novel while the other three compounds (2, 3 and 4) were terpenoids previously isolated from the roots and flowers of the UD[19].
OH
Figure 1: Structure of urticol
Compound 1 was characterized using physical and spectroscopic data as 1’-Hydroxy-1’,4’,7- trimethylcyclohex-2-ylnaphthalene and given the trivial name urticol.
The potential stimulatory effect of compounds 1, 2,
3, 4 and pioglitazone in hepatic glucose uptake were examined at 50 µM and 100 µM concentrations. Glucose uptake increased with time in all the treatment groups and controls, and in both treatment doses.
At 50 µM dose, compound 2 down regulated the uptake of glucose by rat hepatocytes as compared to the untreated control group at all the experimental time intervals. The mean values for compound 2 treatment groups differed significantly with those of
immunosuppressive drugs, with significant side effects, to stop the immune system from rejecting the transplanted islets [10, 18]. Therefore, plant materials are considered to be the alternative sources for finding new leads for hypoglycemic agents. It has been found out that the nettle has antihyperglycemic activity. However, its mechanism of action has not been known[1]. We hypothesized that understanding the structure of the active antihyperglycemic components of UD leaves extract will assist in gaining insight into the probable mechanism of action of the extract which in turn will lead to development of more potent drugs and better methods of diabetes therapy. The present study intended to characterize urticol, isolated from UD[19] as a pontent antihyperglycemic agent its stimulatory effect on hepatic glucose uptake by comparison with those of known agents like pioglitazone.
untreated control at p<0.05. For example, compound
2 was found to decrease glucose absorption in hepatocytes by 36.2 % and 25 % at 15th and 30th minutes respectively when compared to the untreated control (data not shown). Also, compound 4 displayed comparable activity with those of compound 2. It significantly down regulated the uptake of glucose by hepatocytes with respect to untreated control (p<0.05). Compound 1 and pioglitazone treatment groups were found to have mean values comparable to those of untreated control at all experimental time intervals. Therefore,
compound 1 and pioglitazone at 50 µM doses were found to be non-bioactive (data not shown).
However, when the dosage was increased to 100
µM, all the treatment compounds (2, 3 and 4) except compound 1 did not show any potency to increase glucose uptake in primary rat hepatocytes. Compounds 2 and 4 significantly down regulated glucose uptake in rat hepatocytes with respect to the untreated control although glucose uptake increased significantly between experimental time intervals within each of the treatment groups. Compound 3 mean values were comparable with those of the untreated control. Compound 1 was found to significantly upregulate the uptake of glucose in primary rat hepatocytes when compared to the untreated control. Figure 2 shows that the uptake of
glucose in hepatocytes increased significantly with
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 279
ISSN 2229-5518
time for compound 1 treatment group. Although the difference between urticol and pioglitazone for the active dose (100 µM) was insignificant at p<0.05, the mean difference at the 60th minute interval shows that compound 1 increased glucose uptake in hepatocytes by 12.70 % versus pioglitazone. The results obtained demonstrate that the bioactivity of urticol is stronger than that of pioglitazone (member of thiazolidinediones) in stimulation of glucose absorption in rat hepatocytes hence UD, in part, improves diabetes by enhancing glucose uptake in hepatocytes. The observed effect for compound 1 serves to increase liver glucose storage and reduce blood glucose level and hence could possibly reverse/prevent insulin resistance.
As stated earlier, insulin resistance in the liver
reduces glucose storage and this effect serves to elevate blood glucose level and hence progression to
T2DM. Also, increased free fatty acids are associated with hepatic insulin resistance resulting in increased gluconeogenesis [20]. The improved glucose uptake observed in this study due to compound 1 could possibly reduce free fatty acids in the liver and hence hepatic insulin sensitivity which would decrease endogenous glucose production. Insulin stimulates glucose uptake in the liver of both insulin-sensitive and insulin-resistant subjects [21]. Besides, insulin has been shown to upregulate glucokinase transcription [22, 23] and glycogen synthase activity [24] and to inhibit glucose-6-phosphatase [25] and glycogen phosphorylase [24] in hepatocytes ex vivo. Compound 1 may mimic insulin action (insulin mimetic) ex vivo and could possibly upregulate glucokinase transcription and other previously mentioned enzymes just as insulin does.

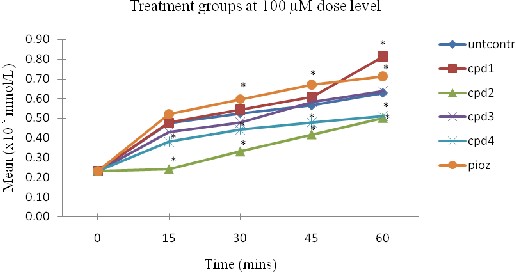
Figure 2: Glucose variations with time for treatment groups at 100 µM dose
The values were presented as Mean ± SD of the mean for n=3. *Mean values considered statistically different at
p<0.05 versus untcontrol. Untcontrol – abbreviates untreated control, cpd (1-4) – compound (1-4) from UD leaves and pioz – pioglitazone.

The liver plays a crucial role in glucose metabolism and is an important regulator of glucose levels in plasma [26]. The liver and the pancreatic
β−cells, as compared to other tissues involved in
glucose homeostasis, can sense and respond to blood glucose levels [20]. After glucose ingestion, the liver suppresses its basal rate of glucose production and takes up approximately one-third of the glucose in
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 280
ISSN 2229-5518
the ingested meal. Suppression of hepatic glucose production and augmentation of hepatic glucose uptake, collectively, account for the maintenance of nearly one-half of the rise in plasma glucose concentrations following ingestion of a carbohydrate meal. Hepatic glucose uptake serves to decrease postprandial glucose and hence prevent progression of hyperglycemia (in the state of hyperinsulinemia) to insulin resistance. Also, the observed comparable effect of compound 1 to that of pioglitazone in the present study may imply that urticol could increase the expression of PPAR-γ as earlier reported on the crude dichloromethane extract of UD leaves by Christensen [27]. The improved insulin sensitivity may be achieved by either systemic insulin sensitivity or by direct action of PPAR-γ on the transcription of genes involved in glucose disposal [20]. Therefore,
EXPERIMENTAL
General experimental procedures
Hepatocytes viability was done by trypan blue test using hemocytometer and then counted using inverted microscope (Olympus BX 41). Statistical analysis was done using SPSS V16, Tukey posthoc one-way analysis of variance (ANOVA) and T-test, and graphs were generated using Microsoft Excel software.
In vitro model for the assay of the compounds from UD
In order to determine the antihyperglycemic effects
of UD compounds isolated, an ex vivo model was designed to investigate on the effects of these compounds on hepatic glucose uptake using freshly isolated primary rat hepatocytes. The hepatocytes were isolated according to the protocol by Prajapati and Patel (2011) and River (2012) and the cells were treated in the presence or absence of UD compounds and the conventional drug for diabetes (pioglitazone). The amount of cellular glucose was determined according to the protocol by Heim and co-workers (2002) using DNSA colorimetric method of assay.
Reagents for the assays
Bicarbonate buffer (BB) with glucose, HEPES buffer pH 7.4 and without calcium chloride suitable for cell culture; 1000 mL bubbled in 5 % CO2 for 20
minutes, sterile filtered and stored at 4 oC - 50 mL at
37 oC per rat in two 25 mL aliquot, 50 mM EDTA solution; 500 mL, 0.11 M CaCl2 ; 250 mL, MEM (100 mL) and DMEM (500 mL) each supplemented with 10
% FBS, 4 mM Glutamine and Pen/strep, 0.25 %
there is a possible amplification of stimulated insulin secretion by urticol as PPAR-γ agonist.
CONCLUSION
Biological assay of urticol using its stimulatory effect on hepatic glucose uptake by freshly isolated primary rat hepatocytes model showed that the compound has potent antihyperglycemic activity greater than the conventional drug pioglitazone at
100 µM dose level. This enhanced glucose uptake activity of urticol ex vivo explains, in part, the role of UD in improving the state of hyperglycemia and insulin resistance. In general, there is induction of glucose uptake by urticol, and so UD, in freshly isolated primary rat hepatocytes in improving the state of hyperglycemia and hence T2DM.
Trypsin solution; 200 mL, Phosphate buffered saline
(PBS) pH 7.4; 500 mL, Perfusion buffer 1; 250 mL BB
+ 0.5 mL 50 mM EDTA, Perfusion buffer 2; 250 mL BB + 343 µl 0.11 M CaCl2 , D-Glucose. 250 mL of lysis buffer (50 mM Tris HCl, pH 7.4, 2 mM EDTA and 0.1 % SDS).
Animals
Two twelve-week old male albino rats, and of about the same weight, obtained from Vet labs (Kenya) were used for the study. They were acclimatized for at least two weeks with free access to food and water in well ventilated room (25 oC) under a 12 hour light 12 hour dark cycle, and were fasted for 12 hours prior to the experiments.
Isolation of rat hepatocytes
The rats were anesthetized using chloroform and sprayed with 70 % ethanol. A horizontal incision was then made on the ventral side near to the diaphragm and perfusion carried out using 30 mL of perfusion buffer 1 at 37 oC followed by 30 ml of perfusion 2. During perfusion, the abdominal area was washed carefully with PBS at 37 oC to flush clotting blood pools and to keep the hepatocytes warm. The liver changed colour from red to light. After perfusion, the liver was excised, placed in chilled Petri dishes containing PBS and minced with scissors in the tissue culture hood. The small pieces were transferred into a
250 mL conical flask where 20 mL 0.25 % Trypsin solution was added to cover them. The content in the flask was magnetically stirred for about 1 h (until
turbid), filtered using muslin cloth and the filtrate
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 281
ISSN 2229-5518
transferred into two chilled 25 mL conical tubes where they were centrifuged (50 g) for 4 minutes at 4 oC. The supernatant was decanted and the pellet washed three times with chilled PBS to remove trypsin. Thereafter,
10 mL of cold bicarbonate buffer was added and the cells redispersed by gently pipetting in up and down fashion using a 25 mL pipette. The 25 mL conical tubes were filled to 20 mL with cold BB and centrifuged (50 g) for 3 minutes. The supernatant was decanted and 20 mL of bicarbonate buffer added to wash the cells, and resuspended by gently pipetting. Again, centrifuged for 3 minutes and the supernatant decanted. Finally, the cells were resuspended in 10 mL low glucose minimum essential media (MEM) at 37 oC and the cell viability determined.
Assay of cell viability
Cell viability was determined using Trypan blue test at the ratio of 1:3, cells to trypan blue reagent in a 2 mL vial. The mixture was then loaded onto the hemocytometer and the cells counted using inverted microscope. The test revealed that 92 % of the cells were viable while 8 % were not viable.
REFERENCE
1. Das, M., et al., The antidiabetic and antilipidemic activity ofacqueous extract of Urtica dioica L on type 2 diabetic model rats. J. Biol. Sci, 2009. 17: p. 1-6.
2. Tong, P.C.Y. and C.S. Cockrum, Diabetes and its historical and social context: the epidemiology of type 2 diabetes mellitus, in: pick up JC and Williams G (eds)Text book of Diabetes (3rd ed). Blackwell Science Ltd. Massachusets, USA., 2003. 6: p. 1-14.
3. Golalipour, M.J., S. Ghafari, and M.M. Farsi, Effect of urtica dioica extract on quantitative morphometric alterations of liver paranchymal cells in STZ diabetic rats. Int.J.Morphol, 2009. 27(4): p. 1339-1344.
4. Bolkent, S., et al., Immunohistochemical studies on the effect of Aloe Vera on the pancreatic β-cells in neonatal streptozotocin- induced type-II diabetic rats. Egyptian J. Biol., 2005. 7: p. 14-19.
5. Bennett, J. and C.M. Brown, Use of herbal remedies by patients in a health maintenance organization. J Am Pharm Assoc (Wash),
2000. 40(3): p. 353-8.
6. Ryan, E.A., M.E. Pick, and C. Marceau, Use of alternative medicine in diabetes mellitus Diab. Med., 2001. 18: p. 242-245.
Assay of glucose uptake
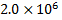
The isolated rat hepatocytes were seeded in 96-wells microtitre plates from corning at the density of cells/mL and incubated in humidified 5 %
CO2 incubator at 37 oC for 3 h to allow for cells attachment. Then, the media in each well was replaced with 200 µl/well complete DMEM supplemented with the compounds from UD leaves and pioglitazone each at the concentrations of 50 µM and 100 µM. The media was collected and the cells washed two times with ice cold PBS. 0.2 mL of lysis buffer (50 mM Tris HCl, pH 7.4, 2 mM EDTA and 0.1 % SDS) was added and the cell suspension pipetted up and down ten times, and then centrifuged at 1000 g for 15 minutes. The amount of glucose in each cell was determined spectrophotometrically, as described by Heim group [26], using dinitrosalicyclic acid colorimetric method (λ = 540 nm) at intervals of 15, 30, 45 and 60 minutes and the results recorded.
7. Gulcin, I., et al., Antioxidant, antimicrobial,antiulcer and analgesic activities of nettle (urtica dioica L.). J.Ethnopharmacol, 2004. 90: p. 205-215.
8. Kavalali, G., et al., Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J Ethnopharmacol, 2003. 84(2-3): p.
241-5.
9. Petlevski, R., et al., Glutathione S-
transferases and malondialdehyde in the liver of NOD mice on short-term treatment with plant mixture extract. Phytother. Res., 2003.
17: p. 311-314.
10. NIDDK., Diabetes. 2010.
11. Onal, S., et al., Inhibition of alpha- glucosidase by aqueous extract of some potent antidiabetic mmeicinal herbs. Prep. Biochem. Biotechnol., 2005. 35: p. 29-36.
12. Daher, C.F., K.G. Baroody, and G.M.
Baroody, Effect of Urtica dioica extract intake upon blood lipid profile in the rats. Fitoterapia, 2006. 77(3): p. 183-8.
13. Ozturk, Y., V.M. Altan, and N. Yildizoglu- Ari, Effects of experimental dabetes and insulin on smooth muscle functions. Pharmacol. Rev., 1996. 48: p. 69-112.
14. Prajapati, R. and R. Patel, Isolation of rat hepatocytes using cold trypsinization method and total RNA isolation using hot SDS/phenol extraction method. Int. J. Curr. Pharma. Res.,
2011. 3(1): p. 23-25.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 282
ISSN 2229-5518
15. Khalil, Z., T. Liu, and R.D. Helwe, Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain, 1999.
79(1): p. 31-37.
16. Farzami, B., et al., Induction of insulin
secretion by a component of Urtica dioica leave extract in perifused Islets of Langerhans and its in vivo effects in normal and streptozotocin diabetic rats. J Ethnopharmacol, 2003. 89(1): p. 47-53.
17. Bnouham, M., et al., Antihyperglycemic activity of the aqueous extract of Urtica Dioica. Fitoterapia, 2003. 74(7-8): p. 677-
681.
18. NIH., Diabetes statistics 2007. 2010.
19. Rono C.K., Muhanji I.C., and M. G.K., Isolation and characterization of urticol from Urtica dioica leaves Int J of Chem Natur Sci,
2015. 3(1): p. 200-204.
20. Kim, H. and Y. Ahn, Role of peroxisome proliferator-activated-γ in the glucose sensing apparatus of the liver and β-cells. Diabetes,
2004. 53(1): p. s60-s65.
21. Iozzo, P., et al., Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET Study. J Nucl Med, 2003. 44(5): p. 682-9.
22. Iynedjian, P., et al., Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon- cAMP system. J. Biol. Chem., 1989. 264: p.
21824-21829.
23. Nouspikel, T. and P.B. Inyedjian, Insulin signalling and regulation of glucokinase gene expression in cultured hepatocytes. Eur. J. Biochem., 1992. 210: p. 365-373.
24. Ortmeyer, H., N. Bodkin, and B. Hansen, Insulin regulates liver glycogen synthase and glycogen phosphorylase activity reciprocally in rhesus monkeys. Am. J. Physiol., 1997.
272: p. E133-E138.
25. Gardner, L., Z. Liu, and E. Barret, The role of glucose-6-phosphatase in the action of insulin on hepatic glucose production in the rat. Diabetes 1984. 42: p. 192-195.
26. Heim, M., et al., Phytanic acid, a natural
peroxisome proliferator-activated receptor agonist, regulates glucose metabolism in prtimary rat hepatocytes. Faseb J., 2002.
16(7): p. 718-720.
27. Christensen, K.B., et al., Activation of PPARgamma by metabolites from the flowers of purple coneflower (Echinacea purpurea). J Nat Prod, 2009. 72(5): p. 933-7.
IJSER © 2015 http://www.ijser.org

