International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015
ISSN 2229-5518
327
Anthocyanin Pigment Identification of Batu Local
Rose Flower as A Natural Colorant to Replace
Harmful Rhodamin B Colorant
Elfi Anis Saati
Abstract— This study aimed at identifying anthocyanin pigment in local rose petal from Batu. Extraction processes were conducted in four- level solvents (aquades-citric acid, aquades-lactic acid, methanol-HCl and Aquades-sulfuric acid). In order to measure the potential amount of anthocyanin pigment, isolation procedure was done with its twice further developers, concentrated HCl: H2O=3:97 and BuOH-HCl (n BuOH: HCl 2N=1:1) resulting in isolate and powdered pigment. The identification of anthocyanin was further conducted by using UV Vis spectrophotometry and FTIR analysis (by malvidin chloride standard). The study result showed that the use of aquades-citric acid and aquades-lactic acid triggered the most apparent anthocyanin pigment identification, as observed by their peak absorbency scale of 0.408 and 0.679 at 513.5-514 nm. The anthocyanin types identified by utilizing spectrophotometry and FTIR analyzes were malvidin glycoside, sianidin glucoside, and pelargonidin glycoside
—————————— ——————————
tential use as natural colorant.
A nthocyanin pigment is a water-soluble pigment that fre-
quently exists in biological diversity in Indonesian nature. It is
commonly represented by red, pink, purple, and bluish colors [1,2,3]. These colors are also likely to be chosen for food and beverage outlook in Indonesian society, including some cos- metic products [4]. However, some news has reported the high frequency of people using non-food colorant such as Rhoda- min B which is in fact harmful for human consumption. This issue has become a major concern especially in higher educa- tion level that holds broad responsibility to develop safe and healthy food technology. Therefore, an exploration of local natural product to replace the dangerous non-food colorant is of urgency. One of which is by extracting the potential of a particular pigment as natural colorant [5].
Rose flower is the most preferred flower and acts as a symbol of love or likeliness; in addition, it is massively grown for dec- orative flower supply both in formal and informal events [6]. Rose is majorly cultivated in Batu city, East Java, where the local rose is crossbred with various roses from different re- gions such as West Java, and Netherland hybrids. Anthocya- nin pigment can be easily tapped in some parts of a plant as in the leaves, fruit [7], root, as well as flower [8,9,10]. The result of the preliminary test conducted on red kana flower [11] which resembles rose at some points revealed that kana flower petals contain anthocyanin pigment. However, very little re- search has been conducted to observe local rose petal sub- stance from Batu; neither did the function of the pigment as a natural colorant which possibly replace harmful non-food col- orant, Rhodamin B [12]. This study aimed at identifying an- thocyanin pigment in local rose petals from Batu and its po-
This study was an experimental study by using simple random sampling. Pigment extraction was conducted by uti- lizing four-level solvents (aquades-citric acid, aquades-lactic acid, methanol-HCl and Aquades-sulfuric acid). The red rose petals (local Batu) as sample were extracted by using: 0.02M of lactic acid, 45gram/300ml solvent, and before being concen- trated by rotary evaporator vacuum (50-60oC) and isolated by twice extraction developers BAA (thick or concentrated HCl:H2O=3:97) and BuOH-HCl (n BuOH:HCl 2N=1:1). The next step was to put small amount of the extract on a thin plague in order to obtain anthocyanin pigment isolates, which led to the further step to thoroughly identify more pigment by UV Vis spectrophotometry [13, 3]. The process was verified by functional group frequency observation by FTIR analysis [14] with its anthocyanin standard of malvidin chloride type.
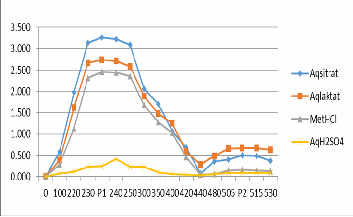
The findings proved that all four solvents used in the ex- traction process could generate anthocyanin pigment resem- bling that of character in band I and II; each represented glikon and aglikon [15]. From Table 1 and Picture 1, it was identified that UV Vis spectrophotometry observation towards maximum absorbance peak in both bands was relevant with the existence of anthocyanin pigment character decisive point, with its maximum absorbance level around 235-244 nm and its aglikon/anthocyanidin around 513.5-518 nm. This study find- ing was in line with Markham [13] asserting that both glikon and aglikon served their functions as glikon flavonoid and
IJSER © 2015
International Journal of Scientific & Engineering Research Volume 6, Issue 4, April-2015
ISSN 2229-5518
328
aglikon [3, 16].
Table 1. Peak absorbance band I and II on pigment filtrate of Batu local rose flower Extraction result with various solvents
Solvents | Glikon Peak maxi- mum absorb- ance (dilu- tion 100x) | Band I at λ (nm) | Aglikon Peak maxi- mum absorb- ance (dilution 10x) | Band II at λ (nm) | Notes |
Aq-sitrat Aq-laktat MetHCl Aq-H2SO4 | 0.3263 0.2737 0.2458 0.0024 | 235 240 244 239.5 | 0.408 0.679 0.157 0.087 | 514 513.5 518 517.5 | The highest Glikon The highest Aglikon |
Notes;

Band I = glikon, Band II = aglikon/ antosianidin
Picture 1. The Distribution Graphic of Glikon (P1) and Aglikon (P2) Peak Absorbance Value of rose flower in various extraction sol- vents.
Aquades solvent and lactic acid produced the highest an- thocyanin pigment peak band II at 0.679 A, whereas aquades solvent and citric acid managed to reach the peak band II at
3.263 A (Table 1). These results asserted that the pigment with aquades and citric acid extract solvents contained higher level of glikon (glucosides); while the highest level of antochyanidin (aglikon) appeared when aquades and lactic acid were put on. These results support the previous studies uncovering that anthocyanin pigment is more stable when it is extracted by moderate polar solvent and with acidic character [17, 8, 15].
The first step of pigment identification was pigment ex- traction (by using lactic acid solvent 0.002M) from the raw local rose petal material as much as 45 gram/300 ml solvent, which had been concentrated by rotary evaporator vacuum (50-60oC). The next step was to observe the pigment by using UV Vis spectrophotometry, especially to identify the maxi- mum absorbance value at 514-518 nm wavelength, based on
the nature of anthocyanin pigment (490-540 nm) [18] and 510-
550 nm [19].
According to its obtained spectrum peak distribution, the identification of functional groups resembling adsorption character was performed to generate a picture of functional group component from the Batu local rose flower petals.
Based on the FTIR analysis, both pigment fractions were expected to have similar molecular structures. It was in line with the previous identification result by thin layer chromato- graphic analysis (KLT) showing that anthocyianidin substanc- es that exist in local red rose petals are sianidin (contain high level of C-OH), malvidin (relatively high level of -O-CH3) and pelargonidin; they serve functional groups at the tip point of ring B which contain hydroxyl and methyl.
The FTIR observation result, after being compared with other samples, indicated that there was similarity in terms of wave frequency range from pigment components of the local red rose from each sample; sample/extract material (crude) of pigment (A), pigment as the result of fractionation I (B) and II (C) as well as pigment powder (D) after being mixed with dex- trin 40%, especially in some sharp bands as the characteristics of anthocyanin pigment band. Those sharp bands appeared on the area of 3400 (A 3442.70; B 3404.13; C 3477.12 and D
3403.20) and the nearby band at 1700 (A 1635.52; D 1639.38; B- C 1635.52) as the signs of phenol and aromatic groups which tied H as an indicator of A and B rings of anthocyanin pigment (aglikon), as well as at Band 1700 (A B 1733.89; C-D 1728.10) as ester group (and carbonyl) that pointed out glikon compounds being tied together by aglikon on band 1722.31 malvidin chloryte [20].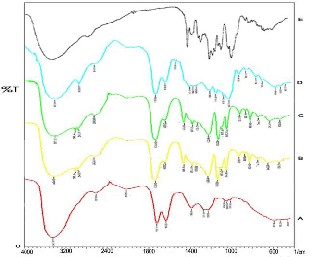
![]()
Picture 2. FTIR analysis of several pigment samples from Batu local red rose (red =A), fractionation result I (yellow
=B) and II (green=C), pigment powder (blue= D), pure malvidin (chlorite)/standard (black= E).
IJSER © 2015
International Journal of Scientific & Engineering Research Volume 6, Issue 4, April-2015
ISSN 2229-5518
329
The band sharpness and the wave frequency spread of local red rose petal pigment sample (fraction I-II/B-C) were relatively close or similar to pure malvidin (as com- parison of IR spectrum peak from maldivin chlorite anto- chyanidin pigment type (Dimitri et.al., 2001), that was the spectrum at 3388 cm–1 wave frequency and some other peaks such as at 1600 or at approximately 1640 cm–1 be- tween 1000- 1300 cm–1. In the first and second pigment fractions, the peak spectrum appeared at 3477.12 cm–1 and 1635.52 cm–1 wave frequency as well as in the range of 1000- 1300 cm–1. Therefore, all samples (4 samples: A, B, C, and D) projected their aglikon/antochyanidin com- ponents that were identified on sharp band at 3442.70 and 1635.52 as the characteristic of phenol and aromatic groups to tie H in A and B rings as aglikon. These com- ponents on the sharp band also represented the existence of ester (carbonyl) group at 1722.31 as the form of glikon compound that has been bounded by aglikon as the standard (external) (D) which suited the result of Dimitri et.al.’s research (2001). He mentioned that antochyanidin pigments that were derived from the same type might produce similar glycosides; Malvidin and Chyanidin. The only difference was the band range which pointed out the location of glikon group substance as an indicator of pigment purity level, when the number of C-H groups found in the band lower than 1000 nm was less than the others. In the band area of 1900-2900, aldehyde com- pounds (CHO and RCHO) were identified as indicators of acylated sugar as the result of fractions I and II; quite contrast with the absence of acylated sugar in malvidin chloride pigment analysis [20].
Anthocyanin pigment extraction from Batu local red rose flower petals was proven to be more effective if it was con- ducted by using aquades with citric acid or lactic acid sol- vents, resulting the peak absorbance of 0.408-0.679Å at 513.5-
514 nm. Types of anthocyanin detected by spectrophotometric and FTIR analyses were malvidin, sianidin, and pelaginidin glycosides.
[1] C.E. Lewis, , J.R.L. Walkerand J.E. Lancaster, “Anthocyanin as Natural Food colours – Selected Aspects”, Food Chemistry. Vol. 58, pp 103 – 109.1997. Bridle, P. and Timberlake, C.F., eds. (Book style and editor)
[2] G.A.F. Henry and J.D. Houghton,”Natural Food Colorants”. Two Edition.
Blackie Academic and Profesional. London. pp 112-120, 1996. (Book style)
[3] Rein, Maarit, “Copigmentation reactions and color stability of berry antho-
cyanins. Food Chemistry Division”, Department of Applied Chemis- try and Microbiology . University of Helsinki. Helsinki.Pp 87, 2005. (Book style)
[4] Wu, Shaowen, C. Ford and G. Horn, “Stable Natural Color Process, Products
and Use Thereof”, Patent application number: 20090246343, 2009.(Patent citation)
[5] F.J. Francis,”Analysis of Anthocyanins”, Markakis, P., (editor). Anthocyanin as Food Colors. Academic Press. New York. pp. 181-207,1982. (Book style and editor)
[6] Rukmana, R. Rose. Publisher Canisius. Yogyakarta. 1995. (Book style)
[7] G.A. Garz’on,K.M. Riedi, and S.J. Schwartz. “Determination of Anthocyanins, Total Phenolic Content, and Antioxidant Activity in Andes Berry (Rubus glaucus Benth)”, J. Food Sci. Vol. 74, Nr. 3 :227-232, 2009.(Journal citation)
[8] L.M.L. Nollet,”Hand Book of Food Analysis”, Two Edition. Marcel Dekker, Inc. New York. Pp 1068, 1996. (Book style)
[9] B.W. Moss, “The Chemistry of Food Colour”, “Colour in Food: Improving Quality”, D.B. MacDougall,eds, Washington: CRC Press, 2002.(Book style with paper and editor)
[10] Kuwayama, Sachiko, S. Mori, M. Nakata, T. Godo, and M. Nakano. Anal- yses of Anthocyanidins and Anthocyanins in Flower Petals of Lychnis senno and Its Related Species (Caryophyllaceae). Bull.Facul.Agric.Niigata Univ.,
58(1):35-38, 2005. .(Journal citation)
[11] E.A. Saati, Mujianto, Susestyarini, R.E.. Function optimalization extract flower of kana as natural colourant and antioxidant by method isolating and characterizing the pigment. Proseding International Reserch Seminar and Exhibition: 2008 Nov 7-8; Malang, Muhammadiyah Unoversitas of Malang. pp 2–4, 2008. (Research Report)
[12] E.A. Saati,Moch. Wachid, “Identification and Characterization of Biologi- cal Pigments Exploration Results Wealth Local as Substitute Color Danger- ous Rodhamin B to Support Availability Food Healthy and Safe, 2012. (Re- search Report)
[13] KR. Markham, “Techniques of flavonoid identification”, Treherne JE,Rubery
PH.,eds, Biological techniques series. New York, NY: Academic Press; pp. 1–
113. 1982. (Book style and editor)
[14] R.M. Silverstein and G.C. Bassaker, “Spectromtric identification of organic coumpounds”, 2nd Ed. John Wiley & Sons, Inc.Pp 393. 1967. (Book style)
[15] J. Li, “Total anthocyanin content in blue corn cookies as affected by ingre- dients and oven types”,Dept. of Grain Science and Industry College of Agri- culture, Kansas University, Manhattan, Kansas, 2009.(Dissertation)
[16] J.J. Macheix, A. Fleuriet and J. Billot,’’Fruit phenolic”, CRC Press. Inc.
Boca Raton-Fla. P 149-237,1990. (Book style)
[17] P. Markakis, “Stability of anthocyanin in foods”, “Anthocyanin as food col- or”,P Markakis,eds. New York: Academic Press. pp. 163-18) ,1982. (Book style and editor)
[18] J.B. Harborne and C.A. Williams,”Advances in flavonoid research
since1992”,Phytochemistry 55: 481–504. 2000. (Book style)
[19] G.Mazza, J.E.Cacace, and CD.Kay,”Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Internat. 87: 129-145, 2004.(Journal ci- tation)
[20] J.M. Dimitri, U.B. Markovi,.Mio, J.Baranac, Z.P. Nedi, “A study of the IR spectra of the copigments of malvin chloride with organic acids. J.Serb.Chem.Soc. 66(7) : 451–462, . 2001. (.(Journal citation)
IJSER © 2015