International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 372
ISSN 2229-5518
T. F. Adepoju1*, S.K. Layokun2, Ojediran, J. O3, Charles, O4
1Chemical Engineering Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria
2Chemical Engineering Department, Obafemi Awolowo University, Ile-Ife, Osun State, P.M.B, 2200055, Nigeria
3Agric&Biosystem Engineering Department, Landmark University, Omu-aran, P.M.B.1001, Kwara State, Nigeria
4Biological Sciences Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria
*Address of Corresponding Author: Chemical Engineering Department, Landmark University, Omu-aran, P.M.B. 1001, Kwara State, Nigeria.
![]()
![]()
Email: avogaIdros200J2@yahoo.coSm, Tel: +234-803E-9404673, Fax: +R234 (36) 232401
…………………………………………………………………………………………………………………………………
Abstract- Response surface methodology (RSM) was employed to optimize L-Phenylacetylcarbinol (L-PAC) production form biotransformation of benzaldehyde via free cell of Saccharomyces cerevisae presence Beta- Cyclodextrin in this work. Specifically, response surface methodology was applied, and the effect of five variables, viz. cell weight, incubation time, acetaldehyde conc., benzaldehyde conc. and β-CD level and their reciprocal were determined. Central composite rotatable design was used to generate 50 individual experiments, which was designed to study the effects of these factors during biotransformation of benzaldehyde to L-PAC. A statistical model predicted the highest biotransformation yield of L-PAC to be
586.938 (mg/100 ml) at the following optimized variables conditions: cell weight of 5.17 g (wet. wt.), incubation time of 74.82 min, acetaldehyde conc. of 1594.05 (µg/100 ml), benzaldehyde conc. of 1300 (mg/100 ml) and β- CD level of 3.20 %. Using these variables under experimental condition in three independent replicates, an actual L-PAC yield of 587.00 (mg/100 ml) was obtained. The physical properties of the produced L-PAC suggested that its could serve as a key intermediate for the synthesis of L-ephedrine, pseudoephedrine, norephedrine, nor-pseudoephedrine as well as adrenaline, amphetamine, methamphetamine, phenylpropanolamine and phenylamine.
Keywords: Biotransformation, Saccharomyces cerevisae, optimization, Response surface methodology, L- PAC.
![]()
…………………………………………………………………………………………………………………………………
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 373
ISSN 2229-5518
methyl ketone also known as L-
Phenylacetylcarbinol (L-PAC) has a molecular formula of C9 H10 O2 . It performance as a key intermediate for the synthesis of L-ephedrine, pseudoephedrine,norephedrine,norpseudoephedr- ne as well as adrenaline, amphetamine, methamphetamine, phenylpropanolamine and phenylamine (Ellaiah and Krishna, 1988; Shukla and Kulkarni, 2002).![]()
• Adepoju T.F is currently pursuing master degree program in Chemical
al., 1988). Biotransformation of benzaldehyde to
optically L-PAC was first experimented by
Neuberg and Lieberman (1921) and the demand for industrial application of this process came about when the chemical synthesis of ephedrine using 1-acetyl phenyl carbinol was patented.
Meanwhile, almost all the literature concerning the synthesis of L-PAC and benzyl alcohol by fermenting yeast deals with yield optimization by free cells (Agrawal et al., 1986;
Cardillo et al., 1991; Zeeman et al., 1992). Studies
IJSER
Engineering at Obafemi Awolowo
![]()
University, Nigeria. He is a Lecturer in the department of Chemical Engineering, Landmark University, Email: avogadros2002@yah.com
• S. K. Layokun is a professor in the Department of Chemical Engineering, Obafemi Awolowo University, Nigeria.
• Ojediran O.J is a senior Lecturer from the Agric & Biosystem Engineering Department, Landmark University
• C. Okolie is Lecturer in biological Sciences Department, Landmark University, Nigeria![]()
L-PAC can be produced by chemical synthesis from cyanohydrins, but the biotransformation route for its production from
benzaldehyde is preferred industrially (Brusse et
revealed that the formation of L-PAC from
benzaldehyde under normal fermentative conditions using yeast, shows that the quantitative conversion of benzaldehyde into L-PAC has never been achieved because of formation of by-products like benzyl alcohol, PAC-diol (Smith and Hendlin,
1953; Gupta et al., 1979; Netraval and Vojtisek,
1982; Agrawal and Basu, 1989). The yeast cannot be used for multiple batches because of the toxic and inhibitory effects of substrate and products (Long et al., 1989; Coughlin et al., 1991).
The use of cyclodextrin always decreased the toxicity of benzaldehyde for bioconversion
using immobilized cells has been reported
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 374
ISSN 2229-5518
(Coughlin et al., 1991; Mahmoud et al., 1990). In
view of these, Vilas et al., 2002, worked on the effect of addition of b -cyclodextrin on biotransformation of benzaldehyde to L-PAC by the cells of Torulaspora delbrueckii in order to increase the optimum yield of L-PAC. Agrwal et al., 1986, worked on the production of L-Acetyl Phenyl Carbinol by yeast employing benzaldehyde as precursor and the results was reported to be
acceptable except that the experiment was not
Kulkarni (2000) worked on L-PAC: biosynthesis
and industrial application. Vrsalovic et al. (2006) carried out research on modeling of transformation processes using numerical method and the process was optimized using the Nelder-Mead algorithm. The numerical values of the parameters were evaluated by fitting the model to the experimental data with the “Scientist” software. The model differential equations were solved numerically by
the fourth order Runge-Kutta algorithm, which is
optimized.
also offered in the same software. The results
IJSER
Production of phenyl acetyl carbinol by
yeast was carried out by Gupta et al., 1978, but no report was available showing high yields of L-PAC production by this mechanism. Biotransformation of benzaldehyde to L-phenylacetylcarbinol (L- PAC) by Torulaspora delbrueckii and conversion to ephedrine by microwave radiation was reported by Vilas et al. (2002). The results obtained were good, except the process condition was not optimized. Shukla and Kulkarni (2001) worked on the process parameters and reusability of the free cell mass of Torulaspora delbrueckii for the production of L-PAC without optimization using
statistical approach. In the same vein, Shukla and
obtained were good except the complexity in the
optimization steps. In this work, biotransformation of benzaldehyde to L-PAC was carried out via the use of free cells of Saccharomyces cerevisiae. To optimize the biotransformation conditions for the production on L-PAC, RSM was applied to determine the effects of five -level-five factors and their reciprocal interactions on the yield of L-PAC.
2.1 Materials
All the chemicals (diethyl ether, anhydrous sodium sulphate, benzaldehyde, acetyladehyde, b-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 375
ISSN 2229-5518
cyclodextrin ((b- CD) etc.) used were of analytical
grade and need no further purification.
2.2 Methods
2.2.1 Microorganisms
Saccharomyces cerevisae used in this study was isolated locally. The culture was consistently maintained on a medium containing 0.4%
dextrose, 1% yeast extract, 1% malt extract, and 2%
agar at pH 7.2 ( Agarwal et al., 1986).
2.2.2 The growth medium
The growth medium for Saccharomyces cerevisae
2.2.4 Biotransformation of benzaldehyde to L-PAC
100 ml of biotransformation medium containing 5% glucose, 0.6% peptone and had pH
4.5 was inoculated with a known weight of cell
mass (biomass) obtained. The reactor was incubated on a shaker at 30 oC and 240 rpm at different time range for adaptation of cells to the medium. Benzaldehyde and acetaldehyde was added and flasks were incubated again for the biotransformation on a shaker at 30 oC and 240
rpm.
IJSER
(Long et al., 1989) contained glucose 2%, peptone
2%, yeast extract 1% and had pH 5.5.
2.2.3 Culture growth
1 ml suspension of cells of the isolate Saccharomyces cerevisae containing 106 cells was inoculated into 9 ml of growth medium and incubated on a rotary shaker at 30 ± 2oC at 240 rpm for 24 h. The obtained culture was inoculated into 100 ml of the same medium and allowed to grow for 24 h. Under the same conditions, cells were harvested by centrifuging at 10, 000 rpm for 15 min at 15 oC. The biomass obtained was washed with water, centrifuged and was used for biotransformation
studies.
2.2.5 Effect of b-cyclodextrin addition on
biotransformation of benzaldehyde
Effect of 0.4 – 1.6% β-cyclodextrin (b-CD) was studied at benzaldehyde and acetaldehyde levels ranging from 500 mg to 1600 mg/100 ml and
400 µl to 1300 µl/100 ml, respectively. The reaction was allowed to take place for 3 h at 30 ± 2oC and
240 rpm. Semi-continuous feeding of different
levels of benzaldehyde and acetaldehyde was also carried out according to design software (Table 1) at different intervals in presence of β -CD.
2.3 Analysis of biotransformation products
Once biotransformation, the medium was centrifuged at 10,000 rpm for 15 min. The
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 376
ISSN 2229-5518
supernatant were extracted three times with equal
volumes of diethylether. The combined extract was dried over anhydrous sodium sulphate and concentrated over a temperature controlled water bath. The residue obtained was dissolved in methanol and subjected to gas chromatography (GC) analysis.
2.4 Gas Chromatography Analysis
The conditions used for GC analysis were as follows- GC model used was Chemito-8510 with Oracle -1 computing integrator. A 4 meter long
L-PAC. Five-level-five-factors design was applied,
which generate 50 experimental runs. This included 32 factorial points, 10 axial points, and 8 central points to provide information regarding the interior of the experimental region, making it possible to evaluate the curvature effect. Selected factors for biotransformation of benzaldehyde to L- PAC were; cell weight g (wet. wt): X1 , incubation time (min): X2 , Acetaldehyde conc. (mg/100 ml): X3 , benzaldehyde conc. (mg/100 ml): X4 and β-CD
level (%): X5 . Table 1 show the independent factors
IJSER
column of 5% OV-17 was used. The injector
temperature and detector temperature (FID) was maintained at 250 oC. Column programming was as follows: 75 oC for 3 min, then 10 oC/ 1 min up to
250 oC and holding time was for 5 min. Retention times of L-PAC was 17 min. The concentration of the compound was determined using peak area method (Shukla and Kulkarni, 1999). The experiment was replicated in triplicate until it was found to be reproducible within ± 3 percent limits.
2.5 Experimental design
and their five levels for Central Composite design,
and the combinations of five independent factors in a Central Composite experimental design.
Depicted in Table 2 also are the L-PAC yields, the predicted yields and the residual values. The effects of unexplained variability in the L-PAC yield response due to extraneous factors were minimized by randomizing the order of experiments.
Central Composite Rotatable Design![]()
Variab le
Sym![]()
bol Coded factor levels![]()
-2 -1 0 1 2
(CCRD) experimental design was employed to
optimize the biotransformation of benzaldehyde to
CW X1 2 3 4 5 6
IT X2 40 50 60 70 80
AC X3 400 700 1000 1300 1600
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 377
ISSN 2229-5518
![]()
31
32
33![]()
CW= Cell weight g (wet. wt), IT= Incubation time 34 (min), AC= Acetaldehyde conc. (µg/100 ml), BC= 35
Benzaldehyde conc. (mg/100 ml)
36![]()
40![]()
41
42
43
44
45
46
47
48
49
50![]()
PV=predicted value (mg/100 ml), Res. = Residual, SR= Standard Runs
2.5.1 Statistical Data Analysis
![]()
The data obtained from biotransformation of benzaldehyde to L-PAC was analysed statistically using response surface methodology (CCRD), so as to fit the quadratic polynomial equation generated by the Design-Expert software version 8.0.3.1 (Stat-Ease Inc., Minneapolis, USA). To correlate the response variable to the independent variables, multiple regressions was used to fit the coefficient of the polynomial model of the response. The quality of the fit of the model
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 378
ISSN 2229-5518
was evaluated using test of significance and
analysis of variance (ANOVA). The fitted quadratic response model is described by Eqn 1:
𝑘 𝑘 𝑘
2
remarkably significant and have very strong effects
on the L-PAC yield witt p< 0.05 (Table 3).
Nevertheless, the linear term X5 with F- value of 3.61x106 and p-value of <0.0001, was the
𝑌 = 𝑏0 + � 𝑏𝑖 𝑋𝑖 + � 𝑏𝑖𝑖 𝑋𝑖 + � 𝑏𝑖𝑗 𝑋𝑖 𝑋𝑗 + 𝑒 (1)
𝑖 =1
𝑖 =1
𝑖 <𝑗
most significant model term. In order to minimize
Where: Y is L-PAC yield (response factor), bo is the
intercept value, bi (i= 1, 2,……… k) is the first order model coefficient, bij is the interaction effect, and bii represents the quadratic coefficients of Xi, and e is the random error.
error, all the coefficients were considered in the design. The results of the second-order response surface model fitting in the form of ANOVA are presented in Table 4. The model F-value (terms used to estimate effects) of 2.156 x105 with low p-
value (<0.0001) implied a high significance for the
Table 2 IshowsJthe codSed factors ER
considered in this study with L-PAC yield, predicted value as well as the residual values obtained. Design Expert 8.0.3.1 software was employed to evaluate and determine the coefficients of the full regression model equation and their statistical significance. Table 3 described the results of test of significance for every regression coefficient. Considering the large F- values (the test for comparing the variance associated with all terms with the residual variance) and low corresponding p-values (the probability value that is associated with the F -
value for all terms), all the model terms are
regression model (Yuan et al., 2008). The goodness
of fit of the model was checked by the coefficient of determination (R2). R2 should be at least 0.80 for the good fit of a model (Guan and Yao, 2008). In this case, the R2 value of 1.00 indicated that the sample variation of 100% for the L-PAC production is attributed to the independent factors (cell weight, incubation time, acetaldehyde conc., benzaldehyde conc. and β-CD level). The value of the adjusted determination coefficient (Adj. R2 of 1.00) was also identical, supporting a high significance of the model (Akhnazarova and Kefarov, 1982; Khuri and Cornell, 1987) and all p-values were less than 0.05,
implying that the model proved suitable for the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 379
ISSN 2229-5518
adequate representation of the actual relationship
among the selected factors. The lack-of-fit term of
0.8317 was not significant relative to the pure error. In this case, a non-significant lack of fit is good. Hence, the model could be used in theoretical prediction of the L-PAC production. The developed regression model equation describing the relationship between the L-PAC yield (Y) and the coded values of independent factors of cell weight (X1 ), incubation time (X2 ), acetaldehyde
conc. (X3 ), benzaldehyde (X4 ) and β-CD level (X5 )
in Table 5. The low values of standard error
observed in the intercept and all the model terms showed that the regression model fits the data well, and the prediction is good (Table 5). The variance inflation factor (VIF) obtained in this study showed that the 8-centre points are orthogonal to all other factors in the model. The model also proved suitable for the adequate representation of the real relationship among the selected independent factors.
Usually, the three-dimensional (3D)
and their respectiveIinteraJctions is descSribed in Eq. ER
(2).
𝑌(𝑚𝑔⁄100 𝑚𝑙) = 386.51 + 4.80𝑥1 + 7.54𝑥2
+ 13.26𝑥3 + 32.35𝑥4 + 128.61𝑥5
− 2.16𝑥1 𝑥2 − 1.09𝑥1 𝑥3 − 1.97𝑥1 𝑥4
+ 5.91𝑥1 𝑥5 + 0.72𝑥2 𝑥3+ 0.34𝑥2𝑥4
+ 9.22𝑥2 𝑥5 − 0.47𝑥3 𝑥4
+ 15.53𝑥3 𝑥5 + 37.78𝑥4 𝑥5 − 5.21𝑥2
response surface plots are graphical
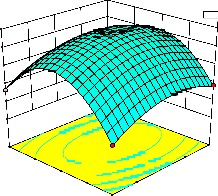
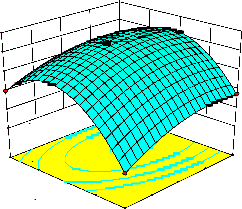
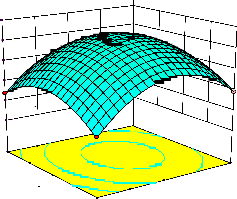
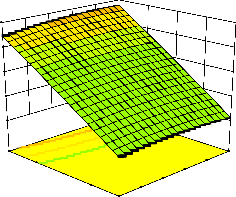
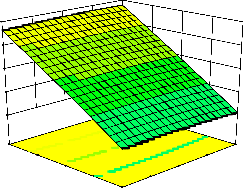
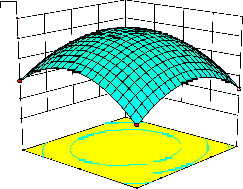
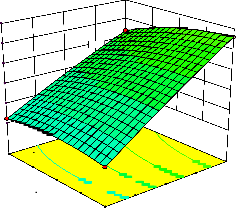
representations of the regression equation for the optimization of the reaction variables, and they are represented in Figure 2. The curvatures’ nature of
3D surfaces in Figure 2a, b, e, f, and h suggested
reciprocal interaction of cell weight with
2 2 2
− 11.22𝑥2 − 13.87𝑥3 − 16.52𝑥4
2
incubation time, cell weight with acetaldehyde
− 5.03𝑥5
(2)
Where Y= 𝐿 − 𝑃𝐴𝐶 𝑦𝑖𝑒𝑙𝑑 (𝑚𝑔⁄100 𝑚𝑙)
All negative and positive values in the
equation shows that the variables have negative and positive effect on the yield of L-PAC production, respectively. The model coefficients
and probability values i.e. coded value are shown
conc., incubation time with acetaldehyde conc.,
incubation time with benzaldehyde conc. and acetaldehyde conc. with benzaldehyde conc., respectively. On the other hand, the nature of curvatures’ of 3D surfaces in Figure 2c, d, g, i, j indicated moderate interactions of cell weight
with benzaldehyde conc., cell weight with β-CD
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 380
ISSN 2229-5518
level, incubation time with β-CD level,
acetaldehyde conc. with β-CD level, and
benzaldehyde conc. with β-CD level, respectively.
The optimal values of the independent factors selected for the biotransformation of benzaldehyde to L-PAC were obtained by solving the regression equation (Equation. 2) using the Design-Expert software package. The optimal conditions for this process were statistically
predicted as X1 = 5.17 g (wet. wt.), X2 = 74.82 (min),
were found to be 170 ± 2 and 251± 2 0C,
respectively.
X3 = 1594.05 (µl/100 ml), X4 = 1300 (ml/100 ml) and
X5 = 3.20 %. The prIedictedJL-PAC yielSd under the ER
above set conditions was 586.938 (mg/100 ml). In
order to verify the prediction of the model, the optimal conditions were applied to three independent replicates, and the average L-PAC yield obtained was 587.00 (mg/100 ml), which is well within the predicted value for the model equation.
In order to ascertain the quality of the L-![]()
PAC produced, the physical appearance was found
to be in powder form, the density was determined
SS= Sum of Square, MS= mean square![]()
to be 1.115 g/cm3, the melting and boiling points
Source
Sum of d
Squares f
Mean
Square
F- value
p-value![]()
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 381
ISSN 2229-5518
Model 5.568 x![]()
105
2 42838.
0 92
2.156 x 105
<0.0001![]()
X5 -5.03 1 0.060 -5.16 -4.91 1.05
Residu al
Lack of fit
5.76 2
9
3.76 2
2
0.20
0.17 0.60 0.8317
Predicted vs. Actual
Pure error
2.00 7 0.29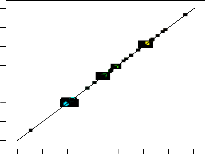
700.00
600.00
Cor
8.568 x 4
500.00
R2 = 100% R2 (adj.) = 100% Std. Dev. =![]()
0.45 Mean = 341.58 C.V. % = 0.13
300.00
200.00
100.00
0.00
4
0.00 100.00 200.00 300.00 400.00 500.00 600.00 700.00
![]()
Actual
580
IJSE570 R
560
550
540
530
520
1.00
0.60
0.20
-0.20
0.00
0.50
1.00
cubationtime(min)
-0.60

-1.00
-1.00
-0.50 Cell weight (wet.wt)
(a)
230
224
218
212
206
200
210.745
-1.00
-0.50
0.00
0.00
-0.50
-1.00
ydeconc. (µg/100ml)
0.50
1.00
1.00
0.50
Cell weight (wet.wt)
(b)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 382
ISSN 2229-5518
240
230
230
222
220
214
210
206
200
198
190
190
-1.00
-1.00
1.00
0.50
0.00
-0.50
-1.00
-0.50
-0.50
cubationtime(min)
-0.50
0.50
0.00
0.00
aldehydeconc. (mg/100ml)
0.50
1.00
(c)
1.00
0.50
0.00
Cell weight (wet.wt)
-1.00
1.00
(f)
Benzaldehydeconc. (mg/100ml)
600
600
500
500
400
300
400
IJSE300 R
200
200
1.00
0.50
0.00
0.00
0.50
1.00
1.00
0.50
0.20
0.60
1.00
ß-CDlevel (%)
-0.50
-1.00
-1.00
-0.50 Cell weight (wet.wt)
ß-CDlevel (%)
0.00
-0.50
-1.00
-1.00
-0.60
-0.20
Incubationtime(min)
(d)
(g)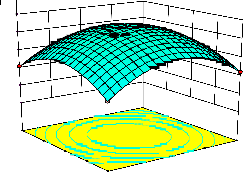
210.745
240
230
220
210.745
245
210
234
200
223
190
212
1.00
0.50
0.00
0.00
-0.50
-1.00
201
190
cubationtime(min)
-0.50
0.50
-1.00
1.00
Acetaldehydeconc. (µg/100ml)
-1.00
-0.50
0.00
-0.50
-1.00
(e)
0.00
zaldehydeconc. (mg/100ml)
0.50
1.00
1.00
Ace0t.a50ldehydeconc. (µg/100ml)
(h)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 383
ISSN 2229-5518
450
400
350
300
250
200
210.745
Beta- Cyclodetrin, indicate that RSM is a good
optimization tools for L-PAC production. The statistical model predicted that the optimal
conditions for the selected biotransformation
-1.00
-0.50
0.00
0.00
0.50
1.00
variables as cell weight of 5.17 g (wet. wt),
incubation time of 74.82 min, acetaldehyde conc. of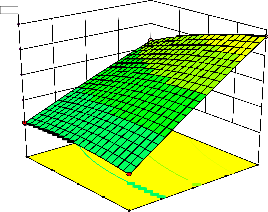
ehydeconc. (µg/100ml)
210.745
600
500
0.50
1.00
-1.00
(i)
-0.50
ß-CDlevel (%)
1594.05 (µl/100 ml), benzaldehyde conc. of 1300 (ml/100 ml) and β-CD level of 3.20 % with an actual L-PAC yield of 587.00 (mg/100 ml). Hence,
this work established the usefulness of RSM for the
400
300
optimum biotransformation of benzaldehyde to L-
IJSEPAC and alsRo the quality of L-PAC produced
200
suggested that it could be used effectively as
-1.00
-0.50
0.00
0.00
0.50
1.00
precursor for the production of L-ephedrine and
dehydeconc. (mg/100ml)
0.50
![]()
1.00
(j)
-1.00
-0.50
ß-CDlevel (%)
D-pseudoephedrine.
The results obtained in this study using response surface methodology to determine the effects of five reaction variables, namely, cell weight, incubation time, acetaldehyde conc., benzaldehyde conc. and β-CD level on biotransformation of benzaldehyde to L-PAC yield
via free cells Saccharomyces cerevisae presence of
The Authors acknowledge the effort of the technical staff of biology and chemistry laboratories of Landmark University. The effort of Chief Technology of ABU, Zaria is highly appreciated.
Agarwal S.C., Basu, S.K., Vora, V.C, Mason, J.R. Pirt, S.J. (1986). Studies on the production of Acetyl
Phenyl Carbinol by yeast Employing
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 384
ISSN 2229-5518
Benzaldehyde as Precursor. Biotechol. Bioeng., 29(6),
783-785.
Akhnazarova, S., Kefarov, V., (1982). Experiment optimization in chemistry and chemical engineering. Moscow: Mir Publishers.
Brusse, J., Roos, E.S., Van Der Gen. A. (1988). Biorganic synthesis of optically active cyanohydrins and acylons. Tetrahedron Letters. 29:
4485-4488.
Cardillo, R., Servi S., Tinti, C. (1991). Biotransformation of unsaturated aldehydes by
Gupta, K.G. Singh, J., Sahani, G. and Dhavan, S.
(1979), Production of phenyl acetyl carbinol by yeasts. Biotechnol. Bioeng., 21(6), 1085-1089.
Khuri, A.I., Cornell, J.A., 1987. Response surfaces:
design and analysis. New York: Marcel Dekker. Long, A., James, P. and Ward, O.P. (1989). Aromatic aldehydes as substrate for yeast and yeast alcohol dehydrogenase. Biotechnol. Bioeng.,
33(5), 657-660.
Mahmoud, W. M., El-Sayed, A.H.M. and Coughlin,
R.W. (1990). Effect of β – Cyclodetrine on
IJSER
micro organisms with pyruvate decarboxylase
activity. Applied Microbiol. Biotechnol., 36(3), 300-
303.
Coughlin, R.W., Mahmoud, W.M. and El-sayed, A.H. (1991). A.H. (1991). Enhanced bioconversion of toxic substances. US Patent. 5173-5413.
Elliah, P and Krishna, K. I. (1988). Effect of aeration and alternating current on the production of Phenylacetylcarbinol by Saccharomyces cerevisae. India Journal of Technology. 26: 509-519.
Guan, X., Yao, H., 2008. Optimization of viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chemistry. 106:
345–351.
production of L-phenyl acetyl carbinol by
immobilized cells of Saccharomyces cerevisae. Biotechnol. Bioeng., 36(3), 256-262.
Netraval, J. and Vojtisek, V. (1982). Production of Phenylacetylcarbinol in various yeast species. Eur. J. Appl. Microbiol. Biotechnol., 16: 35-38.
Neuberg, J and Libermann, L. (1921). Zur kenntnis der carboligase II Muteilung. Biochemische Zeitschnuff. 127, 327-339.
Smith, P. F. and Hendlin, D. (1953). Mechanism of phenyl acetyl carbinol synthesid by yeast. J. Bacteriol., 65, 440-445.
Shukla V.B. and Kulkarni, P.R. (1999). Downstream
processing of biotransformation broth for
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 385
ISSN 2229-5518
recorvery and purification of L-phenyl acetyl
carbinol (L-AC). J. Sci. Indus. Res., 58(8), 591-593. Shukla V.B. and Kulkarni, P.R. (2000). L- Phenylacetylcarbinol (L-PAC): biosynthesis and industrial applications. World Journal of Microbiology & Biotechnology. 16: 499-506.
Shukla V.B. and Kulkarni, P.R. (2001). Process parameters and reusability of the free cell mass of Torulaspora delbrueckii for the production of L-PAC.
World Journal of Microbiology & Biotechnology. 17:
Torulaspora delbrueckii in presence of Beta-
Cyclodetrin. Braz. Arch. Biol. Technol. 45(3), 1-6. Vrsalovic, A. P., Findrik, Z., Zelic, B. (2006). Modeling of the transformation processes. Process Chem. Biotechnol. Engin. 20(3), 227-241.
Zeeman, R.,Netral, J., Bulantova, H., Vodnasky, M. (1992). Biosynthesis of phenyl acetyl carbinol in yeast saccharomyces cerevisiae fermentation. Pharmazie, 47(4), 291-294.
301-306.
IJSER
Shukla, V. B. and Kulkarni, P.R. (2002),
Biotransformation of benzaldehyde in to L- phenylacetylcarbinol (L-PAC) by free cells of Torulaspora delbrueckii in presence of Beta- cylodetrin. Braz. Arch. Biol. Technol., 45(3): 265-268. Vilas, B. S., Virendra, R. M., Bhushan, M. K., Pushpa, R. K. (2002). Biotransformation of benzaldehyde to L-phenylacetylcarbinol (L-PAC) by Torulaspora delbrueckii and conversion to ephedrine by microwave radiation. Journal of Chemical Technology and Biotechnology., 77: 137-140. Vilas, B. Shukla and Pushpa R. Kulkarni. (2002). Biotransformation of benzaldehyde to L-
phenylacetylcarbinol (L-PAC) by free cells of
IJSER © 2013 http://www.ijser.org