International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 1
ISSN 2229-5518
Alkaline Phosphatase Activity during Homogenisation of Hepatopancreatic Tissues of Shrimps using Sodium acetate, KCl solution,
Tris-HCl and Glycine-NaOH buffer.
Kathyayani Puttige, Krishna Prasad Nooralabettu*
Abstract— Hepatopancreat ic tissues of shrim ps, Penaeus monodon, Penaeus indicus, Metapenaeus monocerus, Solenocera Choprai and Parapenaeopsis stylifera was hom ogenized at 3000 rpm/10 min using various buffers such as 0.5M Sodium acetate buffer, 2M KCl solution, Deionised water, 0.1M Tris-HCl buffer and 0.1M Glycine-NaOH buffer of pH 5.5, 7.0, 7.4, 8.4 and 9.5, respectively. Alkaline phosphat ase activities in each of these tissue homogenates were assayed using p-nitrophenylphosphate either as a substrate in respective hom ogenisation buffer or 2-amino 2-m ethyl 1-propanol buffer, as the liberat ion of p-nitrophenol/min at 37 oC/L of hom ogenate. Enzym e activity increased by more than 1.5 folds as the assay buffer changed from respective hom ogenisation buffer to AMP-buffer. Lowest activity was observed in 0.5M Sodium acetate and highest activity was observed in 0.1M Tris-HCl buffer. Increase in pH of the buffer increased the activity of alkaline phosphat ase, but Glycine-NaOH buffer even at pH 9.5 did not favour the activity com pared to Tris-HCl buffer.
Index Terms— Alkaline phosphatase, Shrim ps, Black tiger shrim p, Indian shrim p, W hite shrim p, Brown shrimp, tiny shrim p, Buffer, Hom ogenisation
1 INTRODUCTION
—————————— ——————————
ctivity of alkaline phosphatase is affected by a numerous complex interdependent factors such as
pH, temperature, enzyme concentration, substrate concentration, and effector concentration. Type of the buffer and pH of the buffer is also very important for the activity of alkaline phosphatase. Non-phosphate- containing buffer is required for alkaline phosphatase as phosphate buffer saturates the active site of the alkaline phosphatase and Pi passively hinders the active sites [1]. A wide variety of substrates have been developed to assay its activity. Studies by Hethey et al., [2] suggest that at pH 9, enzymatic conversion of p-nitrophenol phosphate (pNPP) to nitrophenol and phosphate by alkaline phosphatase is higher in a Tris-buffered solution in comparison to Glycine-buffered solution. This difference in alkaline phosphatase activity is stated due to the screening of the substrate by glycine. Even though amino groups are the buffering chains in both Tris buffer and Glycine buffer, the amino groups of Tris is predominantly in the NH2 form, but the amino group of glycine is predominantly in NH+ form between pH 8.6 to
9.1.
——————————————— —
* Corresponding author Krishna Prasad Nooralabettu is currently working as a Professor in the Department of Biotechnology, P. A. College of Engineering, Mangalore, Karnataka, India Pin-574105, PH-
00919448529048. E-mail: lodhariad1@hotmail.com
Co-Author Kathyayani Puttige is currently working as Lecturer,
Department of Biochemistry, K. S. Hegde Medical Academy, Derla katte,
Mangalore, Pin-575018, India
Hence, amine group of Glycine that is positively charged could thus interact with the phosphate group of pNPP that is negatively charged, resulting in the formation of an ionic bond and preventing efficient recognition of pNPP by alkaline phosphatase. However, the amine group of Tris that is neutral would not form an ionic bond with pNPP that in turn allows for efficient recognition of the substrate. Glycine activates alkaline phosphatase when present in very low concentrations (0.1-1 mM), but in the concentrations used in the buffer system (0.01-0.1M) it has an undoubted inhibitory effect [3]. Tojyo [4] reported that increasing the molarity of carbonate buffer or glycine buffer in the assay solution decreased intestinal alkaline phosphatase activity more markedly than enzyme activities of other tissues. Selection of suitable buffer of non inhibitory and suitable pH range during homogenization of hepatopancreatic tissues to isolate in optimum activity, and its effect on the outcome of the alkaline phosphatase assay is very important both scientifically and commercially. Alkaline phosphatase is a phosphomonoester hydrolase that is commonly used as a conjugating enzyme in biological research. Since, there is a considerable demand for enzymes with the right combination of properties for specific application isolation of enzymes with optimum yield and activity from its source using suitable buffer is very important. We have made an attempt to study the effect of the buffer used during the homogenization on
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 2
ISSN 2229-5518
the alkaline phosphatase activity during the assay of hepatopancreatic tissue homogenates.
2 MATERIALS AND METHODS
2.1 Chemicals
Buffers used for homogenization of hepatopancreatic tissues were prepared as per ACS [5]. Assay buffer, 2- amino 2-methyl 1-propanol (AMP) buffer of pH 10.3 was prepared by dissolving 78g of AMP in 500mL of deionised water, and then 200mL of 1M HCl is added, and subsequently made up to 1000mL in 1 L volumetric flak using deionised water. While homogenization mediums such as 0.5M Sodium acetate buffer, 2M KCl solution, 0.1 M Tris-HCl buffer, and 0.1M Glycine-NaOH buffer of pH 5.5, 7.0, 8.4 and 9.5, respectively, are prepared as follows. 0.5M Sodium acetate buffer was prepared by dissolving 4.1g of sodium acetate anhydrous in 1L volumetric using deionised water, and then pH of the buffer was brought down to 5.5 using 0.05M acetic acid that was prepared by adding 2.85mL of glacial acetic acid in volumetric flask and making the volume to 1L using deionised water. 2M Potassium chloride (KCl) solution was prepared by dissolving 149.1g of potassium chloride using deionised water, and pH was adjusted to 7 using 0.1M NaOH solution. 0.1M Tris-HCl buffer of pH
8.4 was prepared by adding 12.111g of the free Tris base to 900mL of deionised water and then titrated with 1M HCl solution until the pH 8.4, and the volume was made up to 1L. Similarly, 0.1M Glycine-NaOH buffer was prepared by dissolving 7.5g of glycine in 900 mL of deionised water and the pH was adjust to 9.5 using concentrated NaOH solution, and finally volume was made up to 1L. The buffers were added with MgCl2 and of ZnCl2 to respective final concentration of 0.1M. All the buffer preparations were filtered and sterilized at 121 oC for 20 minutes. All the chemicals and reagents used were of analytical grade and were obtained from Merck Limited (Mumbai, India).
2.2 Sample Collection
Marine shrimps caught using trawl nets from the Arabian
Sea were obtained from the fishing boats landed in
‘Bunder area’, Mangalore between July and December month. The time elapsed between catching and landing may not exceed over four to six hours. The material was brought in an insulated container after adequately icing them in the proportion of 1:1 shrimp to ice, to the laboratory within two hours. Five different species of marine shrimps such as Penaeus monodon, Penaeus indicus,
Metapenaeus monocerus, Solenocera choprai and
Parapenaeopsis stylifera available along the coastal
Karnataka were identified and used for the present study
[6][7][8]. Hepatopancreatic tissues were packed in plastic
IJSER © 2011 http://www.ijser.org
bags, labeled, frozen at –40 oC, and stored at –20 oC in a deep freezer until further use.
2.1 Homogenisation
The samples were thawed at room temperature of 28 oC. The hepatopancreas and attached tissues were selected, and weighed. The each samples were homogenized in Potter-Elvehjem homogenizer (Rotek Instruments, Kerala,) at 3000rmp/10 min at 4 oC temperature in different lots using 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, 0.1 M Tris-HCl buffer or 0.1M Glycine-NaOH buffer of pH 5.5, 7.0, 7.4, 8.4 and 9.5, respectively, at 1:10 tissue to buffer ratio. The crude homogenate were filtered to remove insoluble and each filtrate was assayed for protein content and alkaline phosphatase activity.
2.3 Enzyme Assay
The procedure used for alkaline phosphatase analysis was basis on that of Bomers and McComb [9]. Substrate was prepared by dissolving 83.5mg of disodium paranitrophenyl phosphate (pNPP) in 1.0ml of 1.5mM magnesium chloride solution and stored at 4 oC. This solution was colorless and used when the optical density was at 410nm< 0.800. While, 10.8mM/L Stock solution of the paranitrophenol (pNP) was prepared by dissolving
150mg of pNP in about 80 mL of 0.25M NaOH solution
and stored at room temperature of 28 oC in brown colored bottle. 54 mM/L working solution of the paranitrophenol (pNP) was prepared freshly by pipetting 0.5mL of pNP stock solution in 100mL volumetric flask and volume was made up to the mark using 0.25M NaOH solution. In one set of experiment, enzyme assay incorporates buffer same as that of the homogenization buffer and in another set of enzyme assay buffer incorporate only 2-amino 2-methyl
1-propanol (AMP) buffer regardless of the types of the buffer used for tissue homogenization. 1.4mL of different buffers were taken, mixed and incubated at 37 oC for
5min. Then 0.05mL of hepatopancreatic tissue homogenates was added. To this mixture, 0.1mL of the substrate was added, mixed and incubated at 37 oC for 15 minutes. Then, 4ml of the 0.25M NaOH was added to each tube in sequence maintaining timed intervals to stops the enzyme activity. Then the content in the tubes were mixed and cooled to room temperature of 28 oC. Colorless pNPP gets hydrolysed by alkaline phosphatase at a given buffer pH and incubation temperature of 37 oC to form yellow colored free pNP, which shows maximum absorbance at 410 nm in a spectrophotometer that was set to zero with the blank. In our alkaline phosphatase assay,
0.05ml of tissue homogenate was mixed with reagent and incubated for 15 minutes and the total volume was made up to 5.55ml. However, the total volume in the case of each standard was 5.0ml. Hence, pNP in mM/L or alkaline phosphatase activity in U/L in the tissue
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 3
ISSN 2229-5518
homogenate = (Test absorbance x 0.027 x 5.55 x 1000) / (Standard absorbance x 15 x 5.0 x 0.05). Alkaline phosphatase activity in U/L is the liberation of 1mM of pNP per minute at 37 oC incubation temperature per liter of tissue homogenate in respective buffers. We made no corrections for the slight variation of molar absorptivity of pNP with pH and (or) buffer concentration. The crude tissue homogenates obtained were assayed for Total protein [10].
3 RESULTS AND DISCUSSIONS
Alkaline phosphatase was isolated from hepatopancreatic tissues of five different species of marine shrimps such as Penaeus monodon (Black Tiger shrimp), Penaeus indicus (Indian white shrimp), Metapenaeus monocerus (Brown
shrimp), Solenocera choprai (Red shrimp) and
Parapenaeopsis stylifera (Tiny shrimp) available along
coastal Karnataka. Suitable working buffer and assay buffer during isolation was selected by homogenizing the hepatopancreatic tissues of Black Tiger shrimp, Indian white shrimp, Brown shrimp, Red shrimp and Tiny shrimp using 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, 0.1M Tris-HCl buffer and 0.1M Glycine-NaOH buffer of pH 5.5, 7.0, 7.4, 8.4 and 9.5, respectively, at 1:10 sample to buffer ratio. We have selected a homogenization speed of 3000 rpm for 10 minutes for further purification as maximum quantity of protein was released by this method. Generally during cell disruption yield is measured in terms of the total protein released, rather than the activity of the enzymes of interest [11]. Alkaline phosphatase assay was performed either using respective homogenization buffer or 2-amino 2-methyl 1-propanol (AMP) buffer. Components and pH of the buffer plays a major role in the alkaline phosphatase activity. Of the three metal binding sites in the active site of the alkaline phosphatase, two zinc ions are shown to play a direct role in catalysis [12]. However, magnesium ion in the active site of the alkaline phosphatase has also shown to play a major role in catalysis [13].
During homogenization of hepatopancreatic
tissues of Black Tiger shrimps, release of protein from the tissues were ranging from 7700 to 8520mg/L of the tissue homogenate using respective buffer. When the tissue homogenates prepared using 0.5M Sodium acetate buffer,
2M KCl solution, deionised water, 0.1M Tris-HCl buffer
or 0.1M Glycine-NaOH buffer, and were assayed using
AMP buffer, alkaline phosphates activity was 59.00±2.16,
92.68±0.94, 96.00±2.31, 122.500±2.89 and 64.00±9.35 U/L, respectively. However when homogenization and assays were performed using 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, 0.1M Tris-HCl buffer and
0.1M Glycine-NaOH buffer, the enzyme activity was
36.50±3.11, 62.68±0.94, 56.00±2.31, 117.75±2.06 and
44.50±1.73 U/L, respectively (Fig.1). When the alkaline phosphatase assay buffer changed from respective homogenization buffer to AMP buffer, catalytic activity of the alkaline phosphatase increased significantly (p<0.01) and the increase was around 1.5 folds, except in the case of homogenate formed using Tris-HCl buffer.
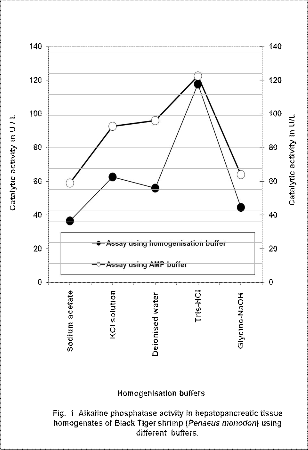
Even though catalytic activity of alkaline phosphatase was increased sharply (p<0.01) as the pH of the buffer used for the homogenization increased, sharp fall in the enzyme activity was observed in the homogenate formed using deionised water and the 0.1 M Glycine-NaOH buffer. Least enzyme activity was observed in tissue homogenate using 0.5 M Sodium acetate buffer of pH 5.5, followed by Glycine-NaOH buffer of pH 9.5, and then the homogenate formed using deionised water. Even though the pH of the buffer increased from 8.4 to 9.5, respectively in 0.1 M Glycine- NaOH buffer and 0.1M Tris-HCl buffer, sharp fall (p<0.01) in the enzyme activity was registered in 0.1M Glycine-NaOH buffer, which may be due to the inhibitory effect of the glycine in the buffer. There is an insignificant (p>0.01) difference in the activity of the alkaline phosphatase activity was registered in samples
homogenized using either the 1M Glycine-NaOH buffer
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 4
ISSN 2229-5518
or 0.5M Sodium acetate buffer. Glycine above 0.01-0.1M concentration is reported to have unfavourable for catalytic activity of alkaline phosphatase [3]. On the other hand at high substrate concentration catalytic activity of the alkaline phosphatase is fairly constant over the range of 7.6 to 9.0, and hydrolysis is very slow at pH value above 9.6 [14].
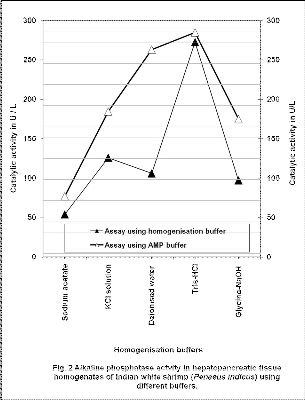
At the end of homogenization of hepatopancreatic tissues of Indian white shrimp, total released protein varied from 8500 to 10800mg/L. During this period, highest activity for alkaline phosphatase was observed in tissue homogenate prepared using 0.1M Tris- HCl buffer and assayed either using AMP buffer or Tri- HCl buffer (Fig. 2). Alkaline phosphatase isolated from Rhizopus microsporus reported to have higher intracellular and extracellular activity in Tris-HCl buffer of pH 8 and 9 [15]. Least alkaline phosphatase activity was observed in the tissue homogenate prepared using 0.5M Sodium acetate buffer and 0.1M Glycine-NaOH buffer, and subsequently assayed using respective buffer. Low activity for alkaline phosphatase in tissue homogenate in
0.5M Sodium acetate buffer assayed using same buffer
may be due to the reversible loss of activity during homogenization and assay. We have register 1.4, 1.4, 2.5 and 1.8 fold increases in the activity of alkaline phosphatase when we have changed the buffers such as
0.5M Sodium acetate buffer, 2M KCl solution, deionised
water, and 0.1M Glycine-NaOH buffer to AMP buffer during assay. Pigretti and Milstein [16] reported that alkaline phosphatase isolated from Escherichia coli undergone reversibly loses its activity below pH 6 and they further stated that the activity also depends on the type of the buffer.
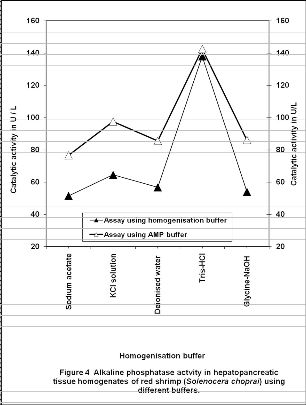
Total protein released from the hepatopancreatic tissues of Brown shrimp during the homogenisation using different buffers varied between 8500 and
12900mg/L. During this period sharp increase (p<0.01) in the activity was observed when the pH of the buffer increased, respectively in 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, 0.1M Tris-HCl buffer, however, the sharp fall (p<0.01) in enzyme activity was observed in sample prepared using 0.1M Glycine-NaOH buffer of pH 9.5. Highest alkaline phosphatase activity was registered in both tissue homogenates prepared using 0.1M Tris–HCl buffer, and assayed using 0.1M Tris–HCl buffer or AMP buffer. On the other hand in those samples both homogenised and assayed using deoionised water and 0.5M Sodium acetate buffer, enzyme activity was 21.00±1.15 U/L and 22.00±2.31 U/L, respectively (Fig. 3). Lower activity in the samples homogenised and assayed using deionised water may be due to the deficiency of the effectors in the homogenate. Zinc and magnecium deficiency negatively effects the activity of the alkaline phosphatase [17], and ionic
strength is necessary for enzyme activity [18].
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 5
ISSN 2229-5518
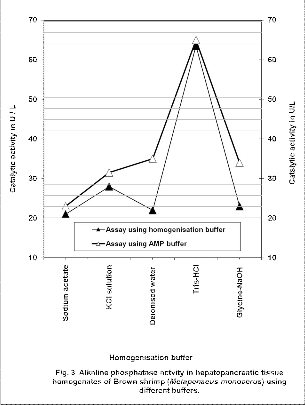
Protein content in the hepatopancreatic tissue homogenates of Red shrimp were between 7700 to 8950 mg/L. During this period, tissue homogenate in 0.1M Tris–HCl buffer registered highest activity of 142.50±4.04
U/L and 138.00±2.00 U/L, respectively in tissue homogenates assayed by AMP buffer and 0.1M Tris–HCl buffer (Fig. 4). Excluding in the case of the homogenate in
0.1M Glycine-NaOH buffer and deiodinised water, catalytic activity increased as the pH of the buffer increased in those homogenates assayed using respective homogenization buffer. However, when the assay buffer changed from respective homogenization buffers such as
0.5M Sodium acetate buffer, 2M KCl solution, deionised water, or 0.1M Glycine-NaOH buffer to AMP buffer, catalytic activity increased by around 1.5 folds.
IJSER © 2011 http://www.ijser.org
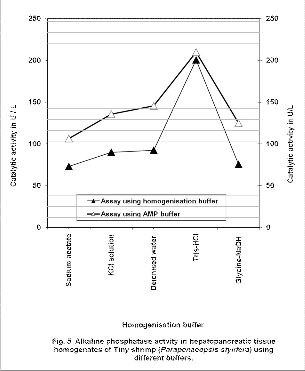
Similarly during the homogenisation of hepatopancreatic tissues of tiny shrimp total protein released using various buffers varied between 7700 to
8950 mg/L. As illustrated in the Figure 5, activity of alkaline phosphatse in both tissue homogenate of tiny shrimp in 0.1M Tris–HCl buffer assayed using 0.1M Tris– HCl buffer and AMP buffer regiserd highest activity. When both the homogenization buffer and assay buffers were 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, or 0.1M Glycine-NaOH buffer catalytic activity was significantly reduced. During this period we have registered increase of catalytic activity by around 1.5 folds when all the respective assay buffers were changed to AMP buffers. Of the various shrimps assayed for alkaline phosphatase activity, white shrimp registered highest activity, and then tiny shrimp, red shrimp, tiger shrimp and brown shrimp.
In this study, during the homogenization of hepatopancreatic tissues of Black Tiger shrimp, Indian white shrimp, Brown shrimp, Tiny shrimp, activity of the alkaline phosphatase in the homogenate increases as the pH increases for all the buffers, so the increase in pH
must directly affect the enzyme and/or substrate. However type of the homogenization media is also very important as lower activity was register in all the species in homogenization medium such as 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, and 0.1M Glycine-NaOH buffer. Similar to the previous results [19] [20] alkaline phosphatase activity was found to be greater
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 6
ISSN 2229-5518
in tissue homogenate prepared and assayed using Tris buffer than in Glycine buffer. Changing the pH of the buffer appears to affect the results of the alkaline phosphatase assay in similar way. The cleavage rate of pNPP by alkaline phosphatase is higher in Tris than in Glycine, as Tris effective nucleophile for the phosphate, after cleavage of pNPP the phosphate remains bound to the active site of alkaline phosphatase that has to be removed in order for the enzyme to bind another molecule of pNPP [21]. This transphosphorylation from the substrate to Tris would accelerate phosphate’s cleavage from the enzyme and clear the enzyme’s active site [2].
4 CONCLUSIONS
In conclusion, hepatopancreatic tissues of Black Tiger shrimp, Indian white shrimp, Brown shrimp and Tiny shrimp during homogenization showed increasing activity for alkaline phosphatase for the substrate pNPP as the pH of the buffer increased. However, alkaline phosphatase activity was registered at higher level in 0.1
M Tris–HCl buffer compared to the 0.1M Glycine-NaOH buffer, eventhough pH of the 0.1M Glycine-NaOH buffer was higher than the 0.1 M Tris –HCl buffer. When assay buffers of changed from the respective homogenisation buffers such as 0.5M Sodium acetate buffer, 2M KCl solution, deionised water, 0.1M Tris-HCl buffer and 0.1M
Glycine-NaOH buffer of pH 5.5, 7.0, 7.4, 8.4 and 9.5,
respectively to AMP buffer alkaline phosphatase activity increased by. However, 0.1 M Tris –HCl buffer of pH 8.4 is found to be favourable for homogenising.
REFERENCE
[1]. L. Zhang, R. Buchet and G. Azzar, “Phosphate Binding in the Active Site of Alkaline phosphatase and the Interactions of 2-Nitrosoacetophenone with Alkaline Phosphatase-Induced Small Structural Changes,” Biophysical Journal, vol. 86, no. 6, pp. 3873–3881, 2004.
[2]. J. Hethey, J. Lai, S.Loutet, M. Martin and V. Tang, “Effects of Tricine, Glycine and Tris Buffers on Alkaline Phosphatase Activity,” Journal of Experimental Microbiology and Immunology, vol. 2, pp. 33-38, 2002.
[3] G.E. Delory and E. J. King, “A sodium carbonate- bicarbonate buffer for alkaline phosphatases,” Biochemistry Journal, vol. 39, no. 3, pp. 245-245, 1945.
[4] Y. Tojyo, “A comparison of the alkaline phosphatases of rat dental pulp, bone, kidney, liver and intestine,” Archives of Oral Biology, vol. 28, no. 2, pp. 103-107, 1983.
[5] American Chemical Society (ACS). Lab Guide, Washington, D.C: ACS, pp. 98-99, 1999.
[6] D.N.F. Hall, “Observations on the taxonomy and biology of some Indo-west Pacific Penaeidae (Crustacea, Decapoda),” Fishery Publications of the (British) Colonial Office, vol. 17, pp. 1–229, 1962.
[7] A.A. Racek, “Littoral Penaeidae from New South Wales and adjacent Queensland waters,” Australian Journal of Marine and Freshwater Research, vol. 6, no. 2, pp.
209-241, 1955.
[8] M.K. Menon, “Identification of marine and inshore prawns of commercial value in India,” Proceedings of the Indo-Pacific Fish Council, vol. 6, no. 3, pp. 345–346, 1956.
[9] G.N. Bomers and McComb, “Measurement of total alkaline phosphatase activity,” Clinical Chemistry, vol. 21, pp. 1988-1995, 1975.
[10] O.H Lowry, N.J., Rosebrough, A.L. Farr and R.J. Randall, “Protein measurement with the folin phenol reagent,” Journal of Biological Chemistry, vol. 193, pp. 265-
275, 1951.
[11] R.H. Cumming and G. Iceton, Cell disintegration and extraction techniques. In: Protein purification technique, 2nd
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 7
ISSN 2229-5518
edn,. Ed. by S. Roe, New York: Oxford University Press, pp. 83-108, 2001.
[12] E.E. Kim and H.W. Wyckoff, “Reaction mechanism of alkaline phosphatase based on crystal structures two metal-ion catalysis,” Journal of Molecular Biology, vol. 218, pp. 449-464, 1991.
[13] B.Stec, K.M. Holtz and E.R. Kantrowitz, “A Revised Mechanism for the Alkaline phosphatase Reaction Involving Three Metal Ions,” Journal of Molecular Biology, vol. 299, pp. 1303-1311, 2000.
[14] R.K. Morton, “The Kinetics of Hydrolysis of Phenyl Phosphate by Alkaline Phosphatases,’ Biochemistry Journal, vol. 65, no. 4, pp. 674–682, 1957.
[15] A.B. Junior, L.H.S. Guimaraes, H.F, Terenzi, J.A. Jorge and Polizei, “Purification and biochemical characterisation of thermostable alkaline phosphatases produced by Rhizopus microsporus var. rhizopodiformis,“
Folia Microbiologica, vol. 53, no. 6, pp. 509-516, 2008.
[16] M.M. Pigretti and C. Milstein, “Acid inactivation of and incorporation of phosphate into alkaline phosphatase from Escherichia coli,” Biochemistry Journal, vol. 94, pp. 106-
113, 1965.
[17] Y.E. Cho, R.A.R. Lomeda, S.H. Ryu, H.Y. Sohn, H.I. Shin, J.H. Beattie and I.S. Kwun, “Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats,” Nutrition Research Practice, vol. 1, no. 2, pp. 113–119, 2007.
[18] B. Asgeirsson, R. Hartemink and J.F. Chlebowski, “Alkaline phosphatase from Atlantic cod (Gadusmorhua) kinetic and structural properties which indicate adaptation to low temperatures,” Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, vol. 110, no. 2, pp. 315-329, 1995.
[19] Yeh, M. F., and Trela, M. 1976. Purification and characterization of repressible alkaline phosphatase from Thermus aquaticus. The Journal Biolological Chemistry,
251: 3141-3139.
[20] A. Banister and R.L. Foster, “Buffer-induced activation of calf intestinal alkaline phosphatase” European Journal of Biochemistry, vol. 113, no. 1, pp. 199-
203, 1980.
[21] M.G. Roig, F.J. Burguillo, A.D. Arco, J.L. User, C. Izquierdo, M.A. Herraez, “Kinetic studies of the transphosphorylation reactions catalyzed by alkaline
phosphatase from E. coli: Hydrolysis of p-nitrophenyl
phosphate and o-carboxylphenyl phosphate in presence of Tris,” International Journal of Biochemistry, vol. 14, pp.
655-666, 1982.
IJSER © 2011 http://www.ijser.org




