International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 890
ISSN 2229-5518
Acid Hydrolysis of Lignocellulosic Content of Sawdust to Fermentable Sugars for Ethanol Production
Adeeyo Opeyemi A*, Ayeni Augustine O, Oladimeji Temitayo E, Oresegun Oyinlola M
Department of Chemical Engineering, Covenant University, Ota, Nigeria ope.adeeyo@covenantuniversity.edu.ng, opeeyo2002@yahoo.co.uk
ABSTRACT: This study evaluates the yield of glucose from acid hydrolysis of cellulosic content of sawdust, at ambient temperature and atmospheric pressure, and the effect of yeast concentration on its subsequent fermentation to ethanol. The method used involves acid hydrolysis of sawdust, with varying acid molarities of 18M, 15M, 10M, 5M and
1M. The product, consisting mainly of simple sugars, was subsequently fermented with varied concentrations of yeast
of 0.5g/20ml, 1g/20ml, 3g/20ml, 5g/20ml and 7g/20ml in order to obtain ethanol. The result obtained shows that there is a gradual increase in the glucose yield with increasing acid molarity from 1M until a critical optimum point is obtained at a high acid concentration of 15M. Beyond the molarity of 15M up to the 18M limit, there exists a decline in the ethanol yield, from the optimum point. The ethanol yield from the fermentation of the resulting fermentable sugars gave the same pattern as the glucose yield irrespective of the yeast concentration used for fermentation. The evaluation of the concentration of yeast on the fermentation of hydrolsed lignocellulosic contents shows that the optimum ethanol yield is obtained at a yeast concentration of 3g/20ml for all the varying acid concentrations. A combination of acid concentration of 15M and yeast concentration of 3g/20ml therefore gives the optimum conditions, at moderate temperature and pressure, for the acid hydrolysis of sawdust’s lignocellulosic content and the fermentation of the resulting product.
Keywords- Acid-Hydrolysis, Ethanol, Fermentation, Glucose, Lignocellulose, Sawdust, Yeast
—————————— ——————————
Ethanol has been made since ancient times by the fermentation of sugars derivable from fruits and grains [1], [2]. Starch from other food sources like tubers such as cassava can also be used. All beverage ethanol and more than half of industrial ethanol is still made by this process using substrate such as corn, sorghum, potato, grape and other cereals and fruits [3]. Ethanol also known as ethyl alcohol, under the organic class of alkanol, is often generally referred to as alcohol [4]. Ethanol is second only to water in solvent and is thus employed in nearly all industries. Presently ethanol is being used as octane boaster for gasoline and a small amount is being consumed as gasohol [5]. Ethanol can be obtained broadly from two sources viz.: those arising from microbial processes (fermentation) of agricultural substrate and those from petroleum based synthetic raw materials. When ethanol is produced by fermentation, glucose
must be the immediate substrate, which is acted upon by the microbial yeast. The yeast enzyme
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 891
ISSN 2229-5518
changes the simple sugars into ethanol and carbon dioxide according to the fermentation reaction equation C6 H12 O6 → 2C2 H5 OH + 2CO2 . There is therefore a pre-requisite of breaking down these compounds, that is, hydrolysis, to produce fermentable sugar, glucose, which is susceptible to fermentation. Starch and cellulose are both polymers of similar based unit monomer, glucose. The glucose molecules are linked together by alpha linkages in starch. The major component in the rigid cell walls in plants is cellulose. The acetal linkage in cellulose is beta which makes it different from starch. Humans are unable to digest cellulose because the appropriate enzymes to breakdown the beta acetal linkages are lacking [6]. Starch can be rapidly depolymerized to yield energy from D-glucose [7]. Production of ethanol from staple starchy foods such as corn, cassava, sorghum leads to a shortage in food, increased demand for arable land for the crops and increased food prices. Emphasis is now shifting to using waste materials and sources that do not bring direct rivalry in human food chain for substrate in ethanol production. One major source of that is cellulosic material. Cellulose is probably the single most abundant organic molecule in the biosphere. Wood is largely cellulose and lignin while cotton and paper are almost pure cellulose. Cellulosic materials are comprised of lignin, hemicellulose, and cellulose and are thus sometimes called lignocellulosic materials. One of the primary functions of lignin is to provide structural support for the plant. Thus, in general, trees have higher lignin contents then grasses. lignin does not contains sugars but encloses the cellulose and hemicellulose molecules, making them difficult to reach. The structural characteristics plus the encapsulation by lignin makes cellulosic materials more difficult to hydrolyze than starchy materials [8]. In addition to glucose (a 6- carbon or hexose sugar), hemicellulose contains pentoses (5-carbon sugars). Hemicelluloses are branched polymers of xylose, arabinose, galactose, mannose, and glucose. Hemicelluloses bind bundles of cellulose fibrils to form microfibrils, which enhance the stability of the cell wall. They also cross-link with lignin, creating a complex web of bonds which provide structural strength, but also challenge microbial degradation [9], [10]. Because lignin is the most recalcitrant component of the plant cell wall, the higher the proportion of lignin the lower the bioavailability of the substrate [11]. Thus, an effective pretreatment is required to loose the cellulose from the lignin seal and its crystalline structure so as to render it accessible for a subsequent hydrolysis [12]. Available pretreatment techniques include acid hydrolysis, steam explosion, ammonia fiber expansion, organosolve, sulfite pretreatment to overcome recalcitrance
of lignocellulsoe (SPORL) [13], alkaline wet oxidation and ozone pretreatment [14]. Besides
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 892
ISSN 2229-5518
effective cellulose liberation, an ideal pretreatment has to minimize the formation of degradation products because of their inhibitory effects on subsequent hydrolysis and fermentation processes [15]. Sawdust is the major waste from the furniture industry. It is primarily fine chippings of wood produced during sawing or cutting. It contains lignocelluloses and certain amount of moisture [16].
Sawdust serves as a cheap substrate for ethanol production, does not distort the human food chain and takes care of the environmental waste. The major difficulty in the hydrolysis of cellulose from wood, to obtain fermentable sugars, lies in separating it from lignin that encloses it and makes it difficult to access. Acid hydrolysis is one of the pretreatment methods used for the lignocellulosic contents to make it susceptible to fermentation to obtain ethanol. Dilute acid hydrolysis requires high temperature and pressure which makes it expensive and inhibit yield. In this work, the effect of varying acid concentration on the hydrolysis of lignocellulosic content from sawdust to produce fermentable sugar is studied. Also, analysis of the yeast concentration on the yield of ethanol from fermentable sugar derivable from corresponding acid-hydrolsed lignocellulosic content is considered.
Hydrolysis of Cellulose
Firstly, the sawdust obtained was pretreated physically with the use of a blender in order to reduce the size of the sawdust and ultimately, the surface area of the material. Next, enough sawdust was added to a 600ml beaker to reach the 100ml mark and weighed. 100mL of 18M sulfuric acid was then added to the sawdust in the beaker. It was ensured that the sawdust was thoroughly (not excessively) covered by the acid. Afterwards, 400ml of distilled water was added to a 1000ml beaker. The sawdust with acid mixture was then poured into the 1000ml beaker with the distilled water. Following this, the pH of the sawdust, acid and water mixture was taken with the pH meter. Water was then added to the mixture to increase the pH to a value between 5.0 and 6.0 (which is conducive for yeast) without exceeding the
800ml mark of the beaker. Since the water could not increase the pH to a value between 5.0 and 6.0, 6N
of NaOH solution was added to the solution with a dropper bottle until the pH was in the desired range. This procedure was repeated for sulfuric acid of concentrations 15M, 10M, 5M and 1M.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 893
ISSN 2229-5518
Determining the Glucose Content
The glucose content that was present in the hydrolyzed sawdust was determined with the use of
Dinitrosalicylic reagent (DNSA) by method Ranken (1984) and a spectrophotometer.
Preparing the DNSA Reagent
The DNSA reagent was prepared by adding 20ml of 2N NaOH to 1g of dinitrosalicylic acid (DNSA) in order to dissolve the DNSA. Then, 50ml of distilled water was used to dissolve 30g of Rochelle salt (NaK tartrate). The NaK tartrate solution was then added to the DNSA solution and the mixture was made up to 100ml with distilled water.
Obtaining the Standard Glucose Curve
Different concentrations of glucose ranging from 0.01mg/ml to 0.1mg/ml were prepared. 2ml of the
DNSA reagent then added to 1ml of the different glucose concentrations. The mixture was heated for
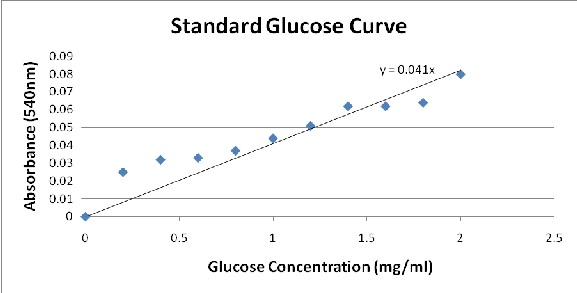
5mins over a water bath, and then cooled in cold water. The absorbance of the sample was obtained with the use of the spectrophotometer at a wavelength of 540nm. The glucose concentration is plotted against the absorbance to obtain a standard curve as show in Figure 1.
Obtaining the Glucose Content
2ml of the DNSA reagent was added to 1ml of a sample. The mixture was heated for 5 mins over a water bath, and then cooled in cold water. The absorbance of the sample was then obtained with the use of the spectrophotometer at a wavelength of 540nm. The glucose concentration was obtained with the use of the standard curve.
Fermentation of the Hydrolyzed Sawdust
After the pH of the mixture was regulated to be between 5.0 and 6.0 which is conducive for the yeast,
250ml of the mixture was poured into a bottle. To prepare an anaerobic condition, 1N of lime solution was prepared and poured into a bottle. Another bottle was obtained and a hole was made. A tube was passed from the bottle with the mixture into the lime solution in the other bottle. 0.5g of yeast was added to 20ml of warm water and about 2ml of the mixture and then shaken for about 5 mins to activate the yeast. This was added to the 250ml mixture in the bottle and then closed tightly. The whole mixture was left for four days to ferment. This procedure was repeated for 1.0g, 3.0g, 5.0g and 7.0g of yeast.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 894
ISSN 2229-5518
Obtaining the Ethanol
After the four days, the mixtures were removed and filtered. The black chunk that was left in the filter paper was the lignin which could be dried and used for fuel. The filtrate contains ethanol and some other impurities. 200 ml of the mixture was then distilled for 45mins. After distilling, the density of the distillate was obtained and compared to the ethanol-water density table to determine the purity of the ethanol obtained.
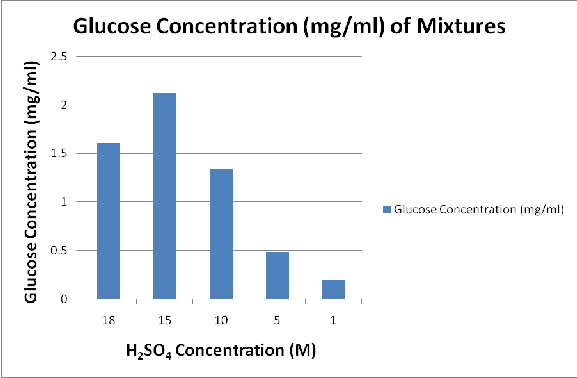
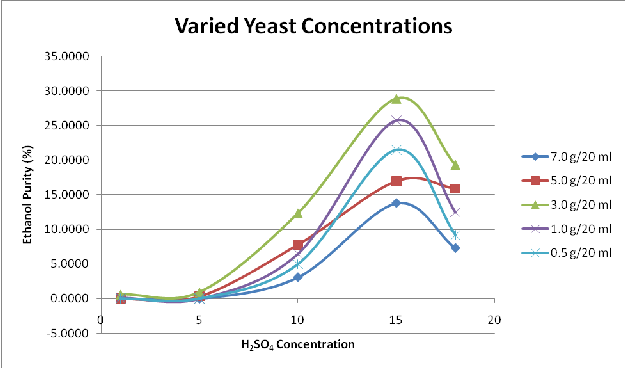
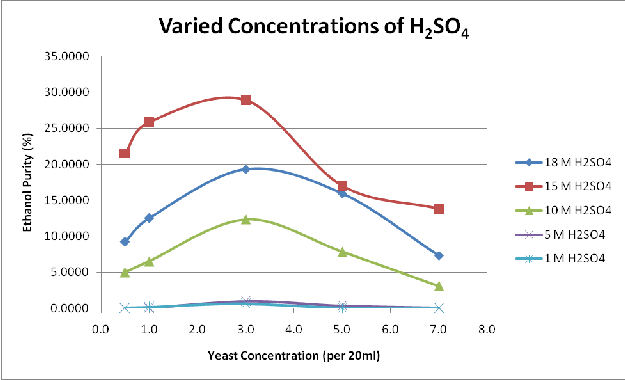
Figure 2 to Figure 4 shows the variation of the yield of fermentable sugars and subsequently ethanol with variations in the acid concentration for hydrolysis and yeast concentration for fermentation.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 895
ISSN 2229-5518
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 896
ISSN 2229-5518
From the results that were obtained, it was observed that the two varied factors, acid concentration and yeast concentration, affect the yield of fermentable sugars and its subsequent fermentation to ethanol. The acid was used to break down the cellulose bonds. Afterwards, the water added caused the broken chains to form glucose and other fermentable sugars that are susceptible to fermentation. Figure 1 shows the standard glucose curve used for the determination of glucose yield.
From Figure 2, it may be observed that due to the acid hydrolysis of sawdust, there is gradual increase in the yield of glucose with increasing acid concentration, from 1M until an optimum critical point is reached at 15M. The reason for the low yield of glucose at low concentration of acid (or dilute acid) was that the low concentration of acid was unable to effectively remove lignin and break down the cellulose bond at atmospheric pressure and room temperature. It was also observed from Figure 2 that beyond the optimum critical point of acid concentration of 15M up to the 18M limit of acid concentration used, there is a decline in the yield of glucose from the optimum critical value. This was because at higher concentration of acid beyond the optimum critical point of 15M, the high concentration of acid leads to the brake down of part of the
fermentable sugars that were formed as a result of the hydrolysis of the lignincellulosic content of the saw dust.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 897
ISSN 2229-5518
This eventually gives rise to the decline in the yield of fermentable sugars at higher concentration of acid beyond the critical point of 15M.
Figure 3 shows the variation of ethanol yield, derived from the fermentation of hydrolysed lignocellulosic content of sawdust, with acid molarities used for hydrolysis. The figure depicts plots of this variation for different concentrations of yeast used for fermentation, ranging from 0.5g/20ml to 7g/20ml. It can be seen that the figure has the same pattern with the glucose yield in Figure 2. The ethanol yield increases with increase in acid molarity and the peak ethanol yield occurs at the acid molarity of 15M with a decline in the yield from the optimum value at concentration beyond 15M. This pattern of ethanol yield is followed by all the yields from the varied yeast concentrations used for fermentation. This also confirm the fact depicted in Figure 2 that the fermentable sugars present in the hydrolysed lignocellulosic content of sawdust increases with increase in acid molarity until the optimum critical point is reached at acid molarity of 15M.
The variation of ethanol yield with concentration of yeast used for fermentation of fermentable sugars is depicted in Figure 4. The fermentable sugars are obtained from acid-hydrolysed cellulosic content of sawdust at varying acid concentrations. It may be observed from the figure that the yield of ethanol increases with increase in the concentration of yeast from 1g/20ml until an optimum critical value is reached at a yeast concentration of
3g/20ml. The ethanol yield thereafter decreases gradually from this optimum critical value as the concentration of yeast rises beyond the critical point at 3g/20ml. This pattern is followed irrespective of the molarity of acid used in the hydrolysis to obtain the fermentable sugars. This shows that for the fermentation of the hydrolysed lignocellulosic content of sawdust, higher concentration of yeast beyond the optimum critical point of 3g/20ml may be counterproductive and inhibit the process of fermentation so as to give rise to a declining ethanol yield.
This study shows that acid hydrolysis, at room temperature and atmospheric pressure, may be used for the hydrolysis of lignocellulosic content of sawdust. The yield from dilute acid hydrolysis of the lignocellulosic content at moderate temperature and atmospheric pressure is small and it increases gradually with increase in the concentration of acid used for hydrolysis until a critical point is reached. The optimum yield of the acid hydrolysis of lignocellulosic content of sawdust, at ambient temperature and atmospheric pressure, occurs at a relatively high critical acid concentration of 15M H2 SO4 . Thus, acid hydrolysis of lignocellulosic content at high concentration of acid may negate the need for high temperature and pressure required for dilute acid hydrolysis in order to obtain substantial yield. The
concentration of yeast also affect the yield of ethanol obtained from the fermentation of fermentable
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 898
ISSN 2229-5518
sugar derived from the hydrolysed lignocellulosic content. The optimum ethanol yield occurs at a critical concentration of 3g/20ml. Negative and positive deviations from this critical yeast concentration gives gradual declining ethanol yield. The optimum conditions for the acid hydrolysis and subsequent fermentation of the lignocellulosic content of the sawdust used therefore occur at a combination of acid concentration of 15M and yeast concentration of 3g/20ml.
REFERENCES
[1] Roach, J. 9,000-Year-Old Beer Recreated from Chinese Recipe. National Geographic News.
[Online]July 18, 2005. [Cited: June 10,
2014.] http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html.
[2] Cocke, William. First Wine? Archaeologist Traces Drink to Stone Age. National Geographic
News. [Online] October 28, 2010. [Cited: June 10, 2013.] http://news.nationalgeographic.com.
[3] Steinkrans, K H. Industrilization of Indigenous Fermented Foods. New York : Marcel Dekker, INC,
1989.
[4] Ranken, M D. Food Industries Manual. 21st. Washington : Kaptain Szabo, 1984.
[5] Austin, G T. Shreve's Chemical Process Industries. New York : Mcgraw-Hill International, 1984.
[6] Ophardt, Charles E. Cellulose. Virtual ChemBook. [Online] 2003. [Cited: June 10, 2013.]
http://www.elmhurst.edu.
[7] Fennema, O R. Food Chemistry. 2nd. s.l. : Marcel Derkker, INC., 1980.
[8] Ethanol from cellulose: Ageneral review. Badger, P C. Alexandra VA : ASHS Press, 2002, pp. 17-
21.
[9] Process considedration in the enzymatic hydrolysis of biomass. Ladisch, M R, et al., et al. 2, s.l. : Enzyme Microb. Technol., 1983, Vol. 5, pp. 82-102.
[10] Substrate availability in the production of composts. Lynch, J M. [ed.] H A Hoitink and H Keener.
1992. International Compositing Research Symposium. pp. 24-35.
[11] Richard, Tom. The Effect of Lignin on Biodegradability. Cornell Composting. Science & Engineering. [Online] 1996. [Cited: June 10, 2013.] http://compost.css.cornell.edu.
[12] Features of promising technologies for pretreatment of lignocellulosic biomass. Mosier, N, et al., et al. s.l. : Bioresour Technol, 2005, Vol. 96, pp. 673-686.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 899
ISSN 2229-5518
[13] Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Zhu , J Y, et al., et al. s.l. : Bioresource Technology, 2009, Bioresource Technology, Vol. 100, pp. 2411-
2418.
[14] Inhibition of ethanol-producing yeast and bacterial by degradation products produced during pre-treatment of biomsass. Klinke, H B, Thomsen, A B and Ahring, B K. 2004, Appl Microbiol Biotechnol, Vol. 66, pp. 10-26.
[15] Fermentation of lignocellulosic hydrolysates for ethanol fermentation. Olsson, L and Hahn- Hagerdal, B. s.l. : Enzyme Microb Technol, 1996, Vol. 18, pp. 312-331.
[16] Effect of Temperature Variation on the Production of Ethanol from Sawdust. Oseghale, Ijogbemeye c and Sideso, M M. 3, s.l. : Research Journal of Applied Science, 2011, Vol. 6, pp.
205-208.
IJSER © 2015 http://www.ijser.org