International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1713
ISSN 2229-5518
Accessing the Potential of Lingocellulosic
Agricultural Waste Biomass for Removal of Ni (II) Metal Ions from Aqueous Streams
Garima Mahajan and Dhiraj sud
Abstract— Acacia saligna pods (ASP), a lignocellulosic agricultural waste material, was used as l ow cost biosorbent for the removal of Ni (II) from aqueous solutions in batch mode. In this study the influences of phase contact time, solution pH, adsorbent dose an d initial concentrations were investigated to optimize the conditions for maximum adsorpti on. The experimental data was analyzed by Langmuir, Freundlich and Temkin isotherm models. The Langmuir and temkin isotherms best represented the measured sorption data. The removal efficiency of the ASP was found to be 93 % under optimized conditions. The kinetics of adsorption of Ni (II) was evaluated by pseudo-first order and pseudo second order kinetic models. The experimental data fitted well with pseudo second order kinetics Results ind icated that the ASP were very effective in removing the selected metal ion from simulated as well as real industrial effluents. Equilibrium was approached within 60 min of the experiment at Ph of 6.0. The selected biosorbent has been analyzed by CHN, for its natural co mposition, FT-IR for identification of contributing functional groups, XRD for its structural properties and SEM for the structural morphology analysis
Index Terms— Biosorption; Acacia saligna pods; Isotherms; Kinetic studies; Nickel
1 INTRODUCTION
—————————— ——————————
Heavy metal ions are the elements in the d-block of the periodic table, or transition metal. Many of them are known to be highly toxic to both humans and other living organisms, with their property of bioaccumulation and to be persistent over times causing remarkable health damage [1]. Heavy metal pollution derives from a number of sources, including industrial effluents, purification of metals, electroplating processes and even leaching of metal ions from the soil into lakes and rivers by acid rain [2]. Unlike organic contaminants, heavy metals do not normally undergo biological decay and thus considered as a challenge for remediation. Many governments have enacted laws to hinder discharging heavy metals into water bodies along with other toxic substances [3]. However these smart metal ions still find their way to water supplies causing serious threat. Accordingly many studies have been done for removal of heavy metals viz; Ion exchange, reverse osmosis, chemical precipitation and advanced treatment processes. All the conventional strategies are too expensive to meet the treatment objectives and permissible limits [4,5,6]. In yesteryears bioremediation by means of waste agricultural materials has gained the momentum due to their potential efficacy and cost suitability [7,8,9,10].

Garima Mahajan Department of Chemistry, Sant Longowal
Insitute of Engineering and Technology, Longowal, Sangrur-
148001, Punjab, INDIA
E-mail: garima8mahajan@hotmail.com
Agricultural waste materials being economic and eco friendly due to their unique chemical composition, availability in abundance, renewability, low cost and more efficiency seem to be a viable option for heavy metal ion remediation. This process of biosorption involves a solid phase (sorbent) and a liquid phase (solvent) containing a dissolved species to be sorbed. Due to high affinity of the sorbent for the metal ion species, the latter is attracted and bound by rather complex process affected by several mechanisms involving chemisorption, complexation, adsorption on surface and pores, ion exchange, chelation, adsorption by physical forces, entrapment in inter and intra- fibrillar capillaries and spaces of the structural polysaccharides network as a result of the concentration gradient and diffusion through cell wall and membrane [8]. In last decade, several investigations have been undertaken for the removal of heavy metal ions ions from wastewater using different low-cost agricultural waste materials [8], such as hazelnut [11], cassia fistula biomass [12], Dalbergia sisso pods [10], waste tea leaves [13], green coconut shells, tea factory waste and saw dust [14,
15, 16]. These agricultural wastes mainly consist of lignin,
cellulose, hemi-cellulose, carbohydrates and some proteins that make them effective adsorbent for heavy metal ions.
Acacia saligna sp. is extensively cultured as a scheme of agro
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1714
ISSN 2229-5518
forestry and social forestry programs in country. The production of acacia has been established in the region for more than 1000 years due to its properties of soil mineral replenishment, medicinal as well as timber value. Like other plant material the pods abundantly growing on the tree are very rich in lignin, cellulose and nitrogen, suggesting its potential utilization for the sequestering of various toxic heavy metal ions and making it a suitable candidate for the process of biosorption. Thus the objective of present study is to investigate the binding of targeted metal ion Ni (II) by ASP in its natural form from simulated aqueous solutions as well as industrial effluents and to study the effect of various factors affecting the efficiency of the process.
2 MATERIALS AND METHODS PREPARATION OF ADSORBENT
Acacia saligna pods (ASP) were collected from local region. Hot
water treatment was given to the ASP for one hour to remove the soluble organic components and sugars, was dried at 1200 C in hot air oven for 24 h, grounded and sieved (150 MICS). To explore the number and positions of the functional groups available for the binding of nickel on to the ASP, FT-IR spectra of native and nickel loaded adsorbent were done on Perkin- Elmer-RX FT-IR system. Structural properties were analyzed with the help of XRD and Surface morphology of the selected biosorbent was done with by means of scan electron microscope (SEM).
PREPARATION OF ADSORBATE
A stock solution of Ni (II) was prepared (1000 mg/L) by dissolving 4.95 g of nickel nitrate in 1 L of de-ionized water. The stock solution was diluted with de-ionized water to obtain the desired concentration range of Ni (II) solutions. The concentration in the test solution was determined spectrophotometrically using Double Array UVVIS Spectrophotometer, (Agilent 8453) at a wavelength corresponding to the maximum absorbance (470 nm) for Ni (II) (DMG method, APHA, 1995). pH of the solutions were adjusted using 0.1 m mol/L HCl or NaOH using Orion 420A pH meter. All the used chemicals were of Analytical grade. The experiments were performed in triplicates and after the simulated experiments; studies were also carried out on real industrial effluents. Composition of the industrial wastewater is given in table 1.
ADSORPTION EXPERIMENTS
Adsorption experiments were carried out by using 100 ml of nickel solution of varying concentration (5-500mg/l) at varying initial pH (1-7 as after pH 7 precipitation of sample was observed) with different agitation speed (50-300), contact time (5-120min) having varying adsorbent dose (50-2500 mg) in 250 ml Erlenmeyer flask at 2510C temperature. Samples were separated by centrifugation at 4000 rpm for 10 min. All experiments were repeated in triplicates and average was taken. Table 1 Composition of indusrial effluent
The removal percentage (R %), defined as the ratio of difference in metal concentration before and after adsorption (Ci – Ce) to the initial concentration of nickel in the aqueous solution (Ci), was calculated using equation (1)
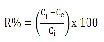 (1)
(1)
3 RESULTS AND DISCUSSION
In terms of their structure, Acacia saligna pods (ASP) are regarded as a lingo-cellulosic agricultural waste material containing high amount of lignin, cellulose, proteins and crude fibers (Table-2). FT-IR analysis of adsorbent before and after sorption of nickel was performed to determine the vibrational frequency changes in the functional groups of the adsorbent.
The spectra of adsorbent were measured in the range of 500 –
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1715
ISSN 2229-5518

4000 cm-1 wave number. The FT-IR of the adsorbent displays a number of adsorption peaks, indicating the complex nature of the studied adsorbent. The presence of fundamental peak at
3340.3 cm-1 indicates the contribution of free and intermolecular
bonded hydroxyl groups. Presence of peak at 2922.7 cm-1 can be assigned to the stretching vibration of the C-H group. The peaks around 1653.3cm-1 correspond to the –C=O group. The strong C- O band at 1056.1 cm-1 due to –OCH3 groups also confirms the presence of lignin structures in Acacia pods. It seems that these
functional groups participate in metal binding process.
Table 2 Structural composition of Acacia saligna pods powder
Structural Components | Percentage |
Carbon | 52.71 |
Nitrogen | 6.23 |
Hydrogen | 5.17 |
Cellulose | 29 |
Hemi-cellulose | 07 |
Lignin | 22 |
Carbohydrate | 16 |
Crude Protein | 14 |
Fiber | 21 |
Ash | 18 |
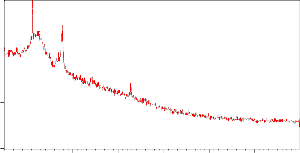
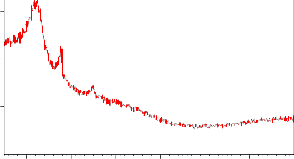
XRD studies reveal the regular pore spacing and pore size of the particles. Further SEM images reveal the surface morphology of the biosorbent giving information regarding the surface texture and porosity of the acacia powder particles. The images explores that the particles have a very narrow size distribution, cylindrical shapes with large availability of cavities acting as a sites for the metal ion adsorption (Fig.1a,b, 2a,b and 3).
Figure 1a,b: SEM of Acacia saligna pods powder in native and
Ni (II) loaded form
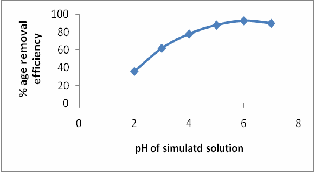
EFFECT OF pH
pH is one of the important controlling parameter in the adsorption process due to its influence on the surface properties of the adsorbent and ionic form of the metal ion in solution. Adsorption experiments were carried out in the pH range of 1-7 keeping all the parameters constant (nickel concentration –
50mg/l, stirring speed – 250 rpm, contact time – 60 min,
adsorbent dose – 1.5g L-1 for ASP, temperature – 250C). The pH of the nickel solution was adjusted after adding the adsorbent. The maximum adsorption of nickel was 93% at pH 6.0. It is observed that with increase in pH from 1.0-6.0 the adsorption efficiency was increased up to 17-93%. Maximum adsorption was occurred at pH 6.0 and hence it was taken as the optimal pH value for further adsorption experiments. On the basis of simulated studies real industrial effluent was taken from the electroplating industry containing Ni metal ion and studies were performed to see the feasibility of mechanism on the real effluents.
Figure 2a, b: XRD image of ASP native and loaded with Ni (II)
Counts

IJSER © 2013
http://www.ijser.org
600
400
200
0
ASP CACACIA POWDER
20 30 40 50 60 70
Position [°2Theta] (Copper (Cu))
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1716
ISSN 2229-5518
Counts
600
400
200
0
Ni LOADEDE ACACIA
20 30 40 50 60 70
Position [°2Theta] (Copper (Cu))
The removal efficiency was found to be near 93% with selected biosorbent. The pH dependence of metal adsorption can largely be related to the type and ionic state of the functional groups present on the adsorbent and the metal chemistry of the solution [17]. At lower pH values the H3O+ ions compete with the metal ions for the exchange sites in the sorbent. As pH increases from acidic range to neutral range, nickel is present predominantly as Ni2+. Moreover the presence of vacant doublet electrons of hydroxyl (-OH-) and carbonyl (C=O) groups the formation of coordination complexes with metal ion also occur.
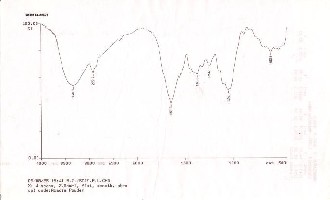
Figure 3: FT-IR image of ASP native
This may be further explained in relation to a complexation effect between the H3O+ and metal ion. At low pH values, the H3O+ ions compete with the metal ions for the binding sites of the ASP, leaving metal ions in solution. As the pH increased, the concentration H3O+ ions decreases, and the sites on the ASP surface become available for the metallic ions in the solution. Our results are found in consistency with other workers that the removal of nickel decreases with the decrease in pH. (Fig-4) Figure 4: Effect of pH on Ni (II) removal by ASP
EFFECT OF STIRRING SPEED
The quantity of the dispersion of the solid in adsorption on a batch reactor is a significant factor as in all processes of mass transfer. Stirring influences the distribution of the aqueous solution. A range of stirring speed from 50-300 rpm was selected for the adsorption experiments at fixed nickel concentration (50mg/l) as well as on real effluent, temp. (250C), pH (6.0), contact time 60 min and adsorbent dose of 1.5 g l-2 of ASP. The adsorption of nickel was low at 50 rpm, and it was observed that with the increase in agitation speed, the adsorption increased. This effect can be attributed to the decrease in boundary layer thickness around the adsorbent particles being a result of increasing the degree of mixing. The optimum value of stirring was found to be 250 rpm as there was no remarkable change in the adsorption between 250 rpm and
300 rpm respectively (Fig-5).
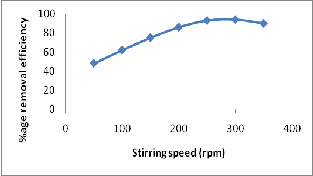
Figure 5: Effect of stirring speed on Ni (II) removal by ASP
EFFECT OF ADSORBENT DOSE
The % adsorption of nickel was studied at different adsorbent doses (50 – 2500 mg) respectively for ASP, keeping Ni (II) concentration (50mg/l), stirring speed (250 rpm), pH (6.0), temperature (250 C) and contact time 60min constant. From the
kinetic study it has been observed that most of the nickel
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1717
ISSN 2229-5518
removal by ASP was achieved in 60 min so these experiments were conducted at 60 min of contact time. There was constant increase in the percentage adsorption of nickel with increase in the adsorbent dose. Increase in the % adsorption with adsorbent dose may be due to the increase in adsorbent surface area and availability of more adsorption sites. (Fig-6)
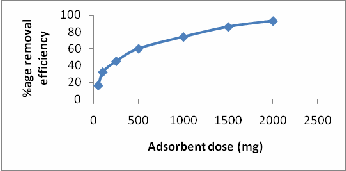
Figure 6: Effect of Adsorbent dose on Ni (II) removal by ASP
EFFECT OF CONTACT TIME/ KINETIC STUDIES
Kinetic studies play a major role for finding the equilibrium during the reaction. Studies on ASP were conducted by varying the contact time from 5–120 min at fixed Ni concentration (50 mg/l), stirring speed (250rpm), temperature (250 C) and pH (6.0). Maximum Ni was sequestered from the solution in 60 min by ASP. The Ni removal was 93% with in 60 min of contact time. It is also found that there is little increase in the adsorption after 60 min of contact time. So keeping these observations in view, 60 min contact time was opted for further experiments with ASP (Fig-7).
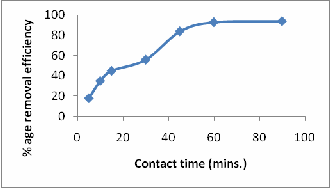
Figure 7: Effect of contact time on Ni (II) removal by ASP
EFFECT OF INITIAL CONCENTRATION
Initial concentration of the work solution is a major parameter as in industrial effluents the amount of heavy metals is at ppm level. So keeping in view the possible range of heavy metals that can be discharged, a wide range of metal ion concentration has been selected. The %age adsorption of nickel with ASP was studied by varying nickel concentration from (5, 10, 25, 50, 75,
100, 250 and 500 mg/l keeping adsorbent dose 2g/l for ASP, stirring speed 250rpm, pH (6.0, temperature 250C and contact time 60 min constant. Higher concentration of metal ion was used to study the maximum adsorption capacity of the adsorbent [18,19]. From the experiments it is found that the
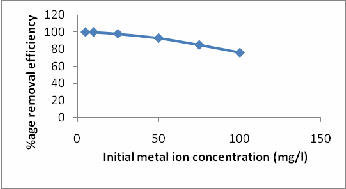
%age nickel adsorption was decreased with increase in initial nickel concentration. But the actual amount of nickel adsorbed per unit mass of the adsorbent was increased with increase in the nickel concentration in the test solution. (Fig 8 and Tab 3). Figure 8: Effect of initial metal ion concentration on Ni (II) removal by ASP
4 ADSORPTION ISOTHERMS
The experimental results obtained for the adsorption of nickel on ASP at constant temperature (25±10C) under pre- defined conditions of pH, adsorbent dose and stirring speed obeyed the Freundlich adsorption isotherm.
Table 3: Adsorption capacity of ASP
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1718
ISSN 2229-5518
Freundlich adsorption isotherm represents the relationship between the amount of metal adsorbed per unit mass of the adsorbent (x/m) and concentration of the metal ion in solution at equilibrium (Ce)
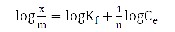 (2)
(2)
Where Kf (l g-1) is an indicator of adsorption capacity and n (dimensionless) indicates the effect of concentration on the adsorption capacity and represents the adsorption intensity (dimensionless). The plot of log (x/m) versus log Ce for various initial concentrations was linear indicating the applicability of the classical adsorption isotherm to this system. The Langmuir isotherm is valid for monolayer adsorption onto a surface containing a finite number of identical sites. The model assumes uniform energies of adsorption on to the surface and no transmigration of adsorbate in the plane of the surface. The Langmuir isotherm is represented by the following equation.
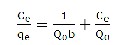 (3)
(3)
Where Ce is the equilibrium concentration (mg/l), qe the amount adsorbed at equilibrium time (mg g-1) and Qo and b are the maximum quantity of metal ions per unit weight of biomass to form a complete monolayer on the surface (mg g-1), whereas b is a constant related to the affinity of binding sites with the
metal ions (l mg-1). The plots of Ce/(x/m) versus Ce are linear
models.
Isotherms | ASP |
Freundlich Isotherm | −1 Kf (l g ) | n | 2 R |
Freundlich Isotherm | 0.643 | 1.016 | 0.946 |
Langmuir Isotherm | Qo (mg g−1) | b (l mg−1) | R2 |
Langmuir Isotherm | 2.82 | 0.0428 | 0.9622 |
Temkin Isotherm | B | A | R2 |
Temkin Isotherm | 8.18 | 1626.4 | 0.9443 |
5 ADSORPTION KINETICS
PSEUDO 1ST& 2ND ORDER EQUATIONS
The kinetics of adsorption is important, as it controls the process efficiency. For evaluating the adsorption kinetics of heavy metals the pseudo-first order equation of lagergren has been used to test experimental data,
ln (Qe-Qt) = lnQe-K1t (5)
where Qe (mg/g) and Qt (mg/g) are the amount adsorbed of heavy metal ions at equilibrium and at time t, respectively, and K1 (min-1) is the rate constant of pseudo 1st order adsorption. The application of this equation to the data of selected biosorbent (data not shown) indicated the inapplicability of the model.
The pseudo 2nd order kinetic model is linearly expressed as [20]
2 + t/Q
(6)
which shows that the adsorption of Ni (II) follows Langmuir
isotherm model. The correlation coefficient (r) values were very high for all the adsorbents, which indicate that the data fitted reasonably well to the Langmuir isotherm in the present adsorption studies. Value of slope found to be lesser than unity implied that significant adsorption took place at low metal ion concentration. To further elaborate the process sorption Temkin isotherm has been employed to the studies. Temkin isotherm describes the behavior of adsorption systems on heterogeneous surfaces. The linear form of Temkin isotherm takes the form:
Qad =RT/b ln A + RT/b ln Ceq (4)
The obtained constants from the plots of Qad Vs. ln Ceq are given in table-4. The appreciably good fitting from the value of R2 does not contradict the applicability of the linear model that suggests the abundance of sites having an equal affinity for adsorption.
Table 4: Parameters obtained from different adsorption
t/Qt = 1/K2Qe e
where K2 (g/mg min) is the pseudo 2nd order rate constant, which can be calculated from the intercept of the straight line obtained from plotting t/Qt with t. Also, the initial sorption rate can be calculated using the relation [19, 20]
Ko = K2 Q 2 (7)
The perfect fit of the experimental data of ASP indicates the applicability of model for the adsorption of heavy metal ions on the selected biosorbent.
6 DESORPTION EFFICIENCY AND REUSABILITY
The regeneration of the biosorbent is one of the key factors in assessing of its potential for commercial applications. Two different desorption agents (1 M HCl and 1 M HNO3) were used to desorbe the Cr (VI) fom the biosorbent. The desorption
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1719
ISSN 2229-5518
efficiency of Ni (II) was found to be 98% and 65% using 1 M HCl and 1 M HNO3 respectively. Therefore, 1 M HCl solution was selected as desorption agent. The reusability of the biosorbent was also tested in10 consecutive sorption-desorption cycles. The results showed that the biosorbent offers potential to be used repeatedly in Ni (II) sequestering process [8].
7 CONCLUSION
Significant adsorption of nickel ions from aqueous solution using the lignocellulosic agricultural waste material was investigated. Various contributing parameters such as contact time, initial metal ion concentration, solution Ph, and adsorbent dose was optimized for maximum removal efficiency. The sorption data fitted well with Langmuir isotherm with high R2 values. The kinetic studies indicated that the pseudo second order model was the best one in describing the kinetics of Ni (II) adsorbed onto pods powder. Further the intraparticle diffusion of nickel ions was not the exclusive rate controlling step. A large number of carbonyl and hydroxyl groups were observed in the FTIR analysis, XRD studies reveal the crystalline structure of the biosorbent and SEM studies showed the presence of various moieties that enhances the adsorption phenomenon. Excellent removal efficiency in its natural form explores the utilization of biosorbent at the commercial scale for small scale industries. Studies on real effluent of an electroplating industry also reveal the potential capability of the biosorbent for the practical utilization in the real and complex matrix, making it of potential commercial use.
REFERENCES
[1] Lentech, 1998, http://www.lenntech.com/heavy- metals.htm
[2] Lef, 1998, http://www.lef.org/protocols/prtcl-
156.shtml
[3] Abdel-Shafy.H.I and Raouf.O.Aly, “Wastewater reuse-risk assessment, decision making and environmental security”, Springer Netherlands,
375-382, 2007.
[4] Bandyopadhyay. A., Biswas. M.N. “Removal of hexavalent chromium by synergism modified adsorption”, Indian J.Environ.Poll 18, 662-671,
1998.
[5] Gupta. V.K., Ali. I., “Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater”, Sep.Purif.Technol.18,
131-140. 2000.
[6] Rao. M, Parwate. A.V, Bhole. A.G, “Removal of Cr and Ni from aqueous solution using Bagasse and fly ash”, Waste Management 22, 821–830,
2002.
[7] Saeed. A., Akhter. M.W., Iqbal, M., “Removal and
recovery of heavy metal from aqueous solution using papayawood as a new biosorbent.Sep.Purif.Technol. 45, 25-31, 2005.
[8] Sud. D, Mahajan. G, Kaur. M.P., “Agricultural
waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: a review”, Bioresour Technol 99, 6017–
6027, 2008.
[9] Mahajan. G. and Sud D., “Modified agricultural waste biomass with enhanced responsive properties for metal ion remediation: a green approach”, Applied water science 2, 299-308,
2012.
[10] Mahajan. G and Sud. D., “Kinetics and
equilibrium studies of Cr (VI) metal ion remediation by arachis hypogeal shells: a green approach”, Bioresources, 6, 3324-3338, 2011.
[11] Cimino G, Passerini A, Toscano G., “Removal of
toxic cations and Cr (VI) from aqueous solution by
hazelnut shell”, Water Res 34, 2955–2962, 2000.
[12] Hanif et al, “Kinetic studies for Ni (II) biosorption from industrial wastewater by Cassia fistula (Golden Shower) biomass”, Journal of Hazardous Materials 145, 501–505, 2007.
[13] Ahluwalia, S.S, Goyal, D, “Removal of heavy metals from waste tea leaves from aqueous solution”, Eng Life Sci 5:158–162, 2005.
[14] Pino G., de Mesquita L., Torem M., Pinto G.,
“Biosorption of heavy metals by powder of green
coconut shell”, Separation Sci. Technol., 41, 3141-
3153, 2006.
[15] Malkoc. E., Nuhoglu. Y., “Investigation of Ni II
removal from aqueous solutions using tea factory
waste”, J. Hazard. Mater., B127, 120–128, 2005.
[16] Shukla. S.S., Yu. L.J., Dorris. K., Shukla A., “Removal of nickel from aqueous solutions by saw dust”, J. Hazard. Mater., B121, 243–246, 2005.
[17] Garg. U.K., Kaur. M.P., Garg. V.K., Sud. D.,
“Removal of Ni (II) from aqueous solution by adsorption on agricultural waste biomass using a response surface methodological approach”, Biores. Technol., 2007.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1720
ISSN 2229-5518
[18] Mohanty. K., Jha. M., Biswas. M.N., Meikap.
B.C., “Removal of chromium (VI) from dilute
aqueous solutions by activated carbon developed
from Terminalia arjuna nuts activated with zinc
chloride”, Chem. Eng. Sci. 60, 3049-3059, 2005.
[19] Ho. Y. S. and Mackay. G., “Kinetic models for the adsorption of dye from aqueous solutions by wood”, J. Env. Sci. Health, 76, 183-187, 1998.
[20] Koynucu. H., “Adsorption kinetics of 3-
hydroxybenzaldehyde on native and activated
bentonite”, Appl. Clay Sci., 38, 279-282, 2008.
IJSER © 2013
http://www.ijser.org









