63% of total soil microarthropods.
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1694
ISSN 2229-5518
Author
Department of Zoology, Nagaland University
Lumami- 798627, Nagaland, India
*Corresponding Author ; e-mail : <minatiborahnlp@gmail .com>
Abstract of the Paper
Abundance and distribution of soil Acarina in two contrasting forest ecosystem was studied for one year at Pathalipam, Lakhimpur, Assam (26°48´- 27°53´ N latitude and 93°42´- 94°20´E longitude, altitude 101 m amsl). Population densities of soil Acarina were more in natural forest site (396.94 x 102m-2 ) than degraded forest (226.32 x 102m-2) contributing 39.26% and 37.51% respectively to the total soil microarthropods. Percentage contribution of Acarina to total soil microarthropods in different depth layers also exhibited similar trend which is reflected as
40.91%, 40.47% and 33.81% in 0-10, 10-20, and 20-30 cm layers respectively. Acarina constituted 44.03%, 31.21% and 25.23% at 0-10cm, 10-20cm and 20-30cm soil layers respectively to the total soil microarthropods. In degraded site, acarine population is comparatively less than the natural forest. Among the sub-orders of Acarina, Cryptostigmata (42.28%) was found to be the most dominant group followed by Mesostigmata (36.62%), Prostigmata (11.58%) and Astigmata (9.53%) in natural forest, while in degraded forest contribution to total Acarina population was shared by Cryptostigmata (65.29%) and Mesostigmata (34.71%,) having no record of the latter two groups. Cryptostigmata, Mesostigmata, Prostigmata and Astigmata were recorded in all soil layers in decreasing order in natural and degraded forest having no record of Prostigmata and Astigmata in degraded forest. While significant correlationship of Acarina with different physical factors were established, but ihe relationship with chemical factors was not found to be significant except for soil potassium in both natural and degraded forest ecosystems.
Key words: Acarina, Abundance, Distribution, Forest ecosystem, Lakhimpur, Assam, India.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1695
ISSN 2229-5518
Soil Acarina was the very small free-living soil and litter microarthropods which was most abundant and dominant group in soil-litter sub system and plays an important role in sustaining forest ecosystem by maintaining the edaphic factors through decomposition and mineralization of leaf litter. The higher concentration of population density of soil Acarina in natural forest attributed to close canopy with vegetation cover, availability of food, accumulation of litter and optimum physico-chemical factors that favour optimum growth of acarine population, while disturbances and lack of canopy in degraded forest site have a negative impact on soil microarthropods community. Soil Acarina was divided into four sub orders viz. Cryptostigmata, Mesostigmata, Prostigmata and Astigmata which are cosmopolitan in distribution and despite their small size of only 0.2-9 mm, their abundance makes them important soil organisms, playing a significant role in decomposition process (Christiansen and Bellinger, 1980). Reduction of vegetation cover and the consequent changes in microclimate has shown negative effects on survival and reproduction of soil microarthropods, however, abundance of soil microarthropods is ecologically important to maintain soil fertility status through decomposition and nutrient cycling for forest restoration (Seastedt, 1984; Badejo and Straalen,
1993; Wardle and Giller, 1996). Doulo and Kakati (2009) recorded higher population of soil micro arthropods in natural site exhibiting conspicuous vertical distribution and seasonal variation in both sites. Hence the present study was carried in natural and degraded forest ecosystem in the abundance, distribution, density and species diversity of soil microarthropods in relation to certain climatic and edaphic factors in this region in Dulung reserve forest, Pathalipam, Lakhmpur district of Assam.
The present investigation was carried out in two adjoining area of natural and degraded forest ecosystem in Dulung Reserve Forest of Lakhimpur district, Assam which lies at 26°48´- 27°53´ N latitude and 93°42´- 94°20´E longitude at an altitude of 101 m above
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1696
ISSN 2229-5518
mean sea level, covering an area of 9900.23 hectre. While the natural forest site comprises of rich vegetation, the vegetation in degraded forest site is comparatively thin vegetation due to human activities and occasional logging of forest tree. The first dominant trees species that form the canopy layer is Keyia assamica (30%). The second dominant trees are Mesua ferrea (15%). The smaller trees are belong to the families of Lauraceae, Euphobiaceae, Araliaceae, Ficaseae and Rubiaceae having 5 to 15m height and shrubs are Alpinia allughas, Alpina pudica L. Calamus erectus (Climber), Calamus viminalis, etc. Very small plants i.e., herbs are Centella asiatica L., Mimosa pudica L., Coix lacrymal- jobi L. Job’s tears,were found abundantly in natural site while degraded forest was mostly represented by less herbs, shrubs etc due to diturbances of human being. Vegetation composition of study area and methodology for extraction of Collembolan population (Modified Tullgren funnel) has beed described by Doulo and Kakati (2009). During sampling soil temperature was measured in situ by usingy soil thermometer and soil moisture was determined by gravimetric method at each different soil depth.
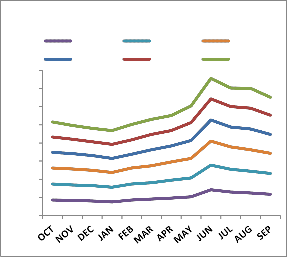
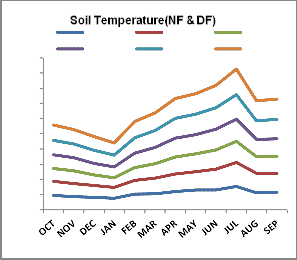
degraded forest. Soil temperature in natural forest exhibited an increasing trend along with the
The average soil temperature and moisture is recorded higher in natural forest than
soil depth which may be due to close vegetation cover and rain that keep the surface cooler than lower region (Fig. 1). The higher soil temperature in top layer in degraded forest except for rainy season may be due to exposure to sun light and low vegetation on the ground than natural forest area. The slight decrease in temperature in top layer than middle layer in rainy season is due to continuous rainfall that helps in raising temporary small vegetation cover that to keep the surface cool. Higher rainfall together with high relative humidity followed by vegetation growth leads to the increase of soil moisture content during rainy season. It is interesting to note while natural forest ecosystem exhibits decreasing trend in soil moisture content during rainy season, the middle layer (10-20 cm) in degrade site retains comparatively higher percentage of soil moisturw than the top layer (0-10 cm) having a trend middle layer> top layer > basal layer (20-
30 cm). However the reverse trend has been observed during dry season (October to May) with increasing and decreasing trend in soil moisture content along with depyh in natural and
degraded forest respectively.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1697
ISSN 2229-5518
Acarina was found to be dominant group comprising 39.26% and 37.51% of the total soil microarthropods in natural and degraded sites respectively. Higher abundance of Acarina among soil microarthropods were also reported in different forest ecosystem (Mitchell, 1977; Wallwork, 1983; Sarkar, 1991). Chitrapati (2002) reported that Acarina comprised 66% and

63% of total soil microarthropods.
200
180
160
140
120
100
80
60
40
20
0
0-10 10-20 20-30
0-10 10-20 20-30
400
350
300
250
200
150
100
50
0
Soil Moisture(NF & DF)
0-10 10-20 20-30
0-10 10-20 20-30
Fig. 1: Monthly variation of soil temperature (ºc) and soil moisture
(%) at different soil layers in natural (N)and degraded(D) forest ecosystem
Doulo (2007) also reported that Acarina comprised 42.4% and 40.8% of the total soil microarthropods in natural and degraded in forest sites respectfully in Lumami, Nagaland. Having been less disturbed and with more favourable microclimatic condition natural site possessed higher Acarina population than degraded site (Aoki, 1967). Hazra (1991) also reported a decrease in population percentage of mites in deforested site as compared to the reserve forest.The Acarina population was recorded to be 1.75 times higher in the natural forest (396.74
x 102m-2) than the degraded site (226.32 x 102m-2). The higher density of microarthropods in
Acarina in upper layer of the soil (0-10cm) was characterized by favourable moisture condition, adequate living space aeration ratio and rich accumulation of organic debris (Peterson, 1980; Hagvar, 1983) had also observed higher density of microarthropod population in the upper layers of the soil. However steep declined in population density in lower layer (20-30 cm) in degraded forest in comparison to natural forest may be attributed to poor living space, insufficient food resources and inimical microclimatic condition which results in maximum migration to top
layer. In both study sites, maximum populationof Acarina during rainy season reached the peak
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1698
ISSN 2229-5518
in August followed by summer and winter season with minimum record during January. Many earlier workers also reported greater abundance of Acarina in the upper soil layer than the lower depth (Wallwork, 1970; Niijima, 1971 and Alfred et al. 1991 and Chitrapati, 2002). Monthly variation of total population density of Acarina exhibited similar trend in both natural and
degraded forest. With the initial record of 24.68 x 102m-2 and 15.27 x 102m-2 during October, the
number decreases to minimum during January (5.90 x 102m-2 and 1.93 x 102m-2) in natural and degraded forest respectively and gradual increases in both natural and degraded forest reaching the peak during August (70.77 x 102m-2 and 40.08 x 102m-2 respectively). In both study sites, maximum population growth during rainy season reached the peak in August. This may be due to favorable, physico-chemical factors i.e. optimum condition of moisture, organic carbon content etc during rainy season as the population buildup of soil microarthropods is influenced by a variety of factors viz., vegetation, soil, climate etc. and their interaction (Narula et al,
1998). Badejo et al. (1997) reported maximum population of Acarina when there was high
moisture content. Loots and Ryke (1966) reported minimum population during winter season.
Forest TyIpe SJeason
SSoil laEyers
RTotal
a =
contribution among the soil layers;
b = contributionof soil Acarina to the total soil microarthropods in each layers
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1699
ISSN 2229-5518
The seasonal vertical distribution pattern also showed a decreasing trend with increase in soil depth in both study sites (Table 1). Soil Acarina in the upper soil layers was primarily found to be influenced by moisture content and secondarily by temperature conditions (Strong, 1967). In the present investigation also similar pattern was observed in the population density of Acarina representing maximum in the upper soil layer at 0-10cm. The seasonal variation of Acarina in both sites in the present investigation was attributed to cumulative effect of all physico-chemical factors rather than a single factor influence. Petersen (1980) and Hagvar (1983) had also shown that the higher densities of micro-arthropods population occurred in the upper layers of the soil. Hattar et al. (1998) and Chitrapati (2002) also reported maximum population of Acarina during rainy season and observed decreasing trend with the on set of winter. Acarina population in natural forest ecosystem showed maximum density in the month of August in 0-10cm during raining season and minimum in 20-30 cm in winter season. Natural forest had higher population density of 19.18%, 68.47% and 123.45% in 0-10cm, 10-20cm and 20-30cm layer respectively than degraded site. The seasonal fluctuation trend of Acarina population density was found to be distinct with maximum during rainy season followed by summer and winter.
Among the sub-orders of Acarina, Cryptostigmata (42.28%) was found to be the
most dominant group followed by Mesostigmata (36.62%), Prostigmata (11.58%) and Astigmata (9.53%) in natural forest, while in degraded forest contribution to total Acarina population was shared by Cryptostigmata (65.29%) and Mesostigmata (34.71%, ) having no record of the latter two groups. Total Acarina and different suborders exhibited maximum concentration in 0-10cm soil layer followed by 10-20 and 20-30 cm respectively in different months (Table 2). Monthly variation of total population density of Acarina exhibited similar trend in both natural and degraded forest. In four different habitats in England, Madge (1965) recorded maximum contribution of Cryptostigmata (90%) followed by very less percentage of Mesostigmata (7%) and Prostigmata (3%) to total Acarina population. Block (1965) found the range of Prostigmata from 0.2 to 15% while the Cryptostigmata ranged from 62 to 94% in six habitats in England. Wallwork (1967) stated that the Cryptostigmata occurred in the greatest
number in coniferous forest soils representing as much as 75% of the total Acarina population.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1700
ISSN 2229-5518
However, Price (1973) reported Prostigmata as the most numerous among the Acarina, contributing 51.3% of the mites fauna, followed by Cryptostigmata (38.5%), Mesostigmata (10.2%) and without any record of Astigmata in pine forest ecosystem. Singh and Pillai (1981) reported that Cryptostimata accounted for 16.99 to 42.21% of the total Acari in different fields at
Table 2: Vertical distribution of different suborders of soil Acarina (Numbers±S.E) x 102m-2) in natural

(N) and degraded (D) forest ecosystem at Lakhimpur
IJSER
b 45.44
c 20.01
19.83
6.19
0 34.71%
0 13.02%
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1701
ISSN 2229-5518
a = Percentage contribution among the soil layers;
b = Percentage contribution to the total soil Acarina in each layer
c = Percentage contribution to the total soil microarthropods in each layer
Varanasi. Alfred et al. (1991), Narula et al. (1998) also reported the maximum density of Cryptostigmata among the Acari and usually Astigmata the least in abundance which was similar to the present finding. Chitrapati (2002) reported highest percentage of Cryptostigmata (48-52%), followed by Mesostigmata (27-29%), Prostigmata (18-29%) and Astigmata (3% only in degraded site) having similar vertical distribution in protected and degraded forest ecosystem at Imphal. Similarly, in the natural and degraded forest ecosystem in Lumami, Nagaland, Cryptostigmata contributed 53.3 to 55.7%, Mesostigmata 25.3 to 31.7%, Prostigmata 13.2 to
14.8% and Astigmata contributed only 1.8 to 4.2% respectively to the total Acarina population (Doulo, 2007). The marked difference in the density among the different groups of Acarina in the present study and others as reported may be attributed to some physiological or behavioral adaptation as group and tolerant ability in different environmental factors (Karppinen, 1955; Ryke and Loots, 1967 and Price, 1973). Cryptostigmatid mites were very sensitive to temperature changes and responded at least in part to diurnal variations in temperature when undertaking their movement (Wallwork, 1961; Madge, 1965). The Oribatids mites acted as indices of specific microclimatic conditions and have conferred species status bioindicators to them (Bhandari and Somani, 1994). Zonal distribution of some species of Acari may be due to selectivity in their food choice (Luxton, 1966). Rajagopal (2011) recorded decrease of mesostigmatid mites during winter season. Sheals (1955) reported that the Mesostigmata and Prostigmata were both predatory and detritus feeders while the Cryptostigmata were primarily detritus feeders and Astigmata were not generally abundant in the soil, although they may occur in local concentrations, particularly in pasture and arable soils. The vertical distributional pattern of these suborders exhibited a decreasing trend with the increasing depth (Fig. 2). The poor availability/absence of Prostigmata and Astigmata in lower vertical layers particularly 20-
30 cm and total absence in degraded sites may be attributed to its less tolerance ability, poor adaptation and limited food habit. Chitrapati (2002) also reported the presence of Astigmata
only in the upper most layer of the soil.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1702
![]()
ISSN 2229-5518
40 Cryptostigmata (a) NF 30 DF 20 10 0 | Prostigmata (c) 8 NF 6 4 2 0 | |
Mesostigmata (b) Astigmata (d) NF 30 8 NF DF 25 6 20 15 4 10 IJS2ER 5 0 0 |
![]()
![]()
80 Acarina total (e)
NF DF
60
40
20
0
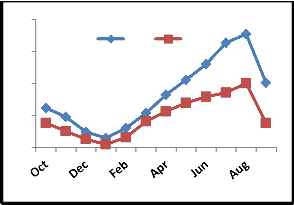
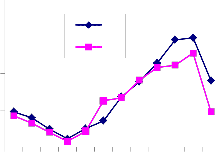
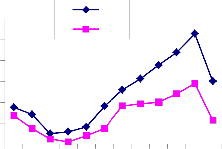
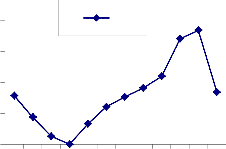
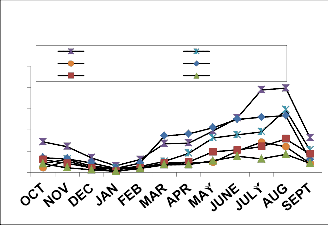
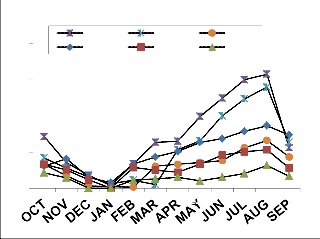
Fig. 2: Monthly fluctuation of Acarina population density in natural) and degraded forest ecosystem (a) Cryptostigmata,(b) Mesostigmata, (c) Prostigmata (d) Astigmata and (e) Total Acarina (Numbers x 102m-2)
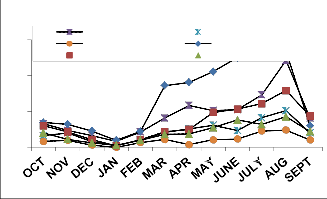
The maximum and minimum population of Acarina were recorded in August and January in both sites at different soil layers respectively. Vertical distribution of Cryptostigmata showed
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1703
ISSN 2229-5518
maximum migration towards the upper soil layer i.e., 0-10cm. Monthly population density of Cryptostigmata was recorded to be maximum in August and the minimum in January of both natural and degraded forest site. Similarly,vertical distribution of Mesostigmata,Prostigmata and Astigmata was also recorded respectively, but no records of Prostigmata and Astigmata in degraded site (Fig. 3). In present investigation Cryptostigmata constituted about 16.59% and
24.49% of the total soil microarthropods in natural and degraded sites respectively. Rykes and Loots (1967) reported 50% of the Cryptostigmata population of the Acari-Collembola total. Singh and Singh (1975) reported only 34% of Cryptostigmata of the total soil fauna. Chitrapati (2002) reported 32% of Cryptostigmata population of the total microarthropods. Doulo (2007) reported 24% and 22% of Cryptostigmata to the total microarthropod in natural and degraded ecosystem in Lumami, Nagaland. Seastedt (1984) reported that many Cryptostigmatid mites were mesophilus and flourish in the moist organic soils under woodland and forest. The other two groups of mites i.e. Mesostigmata and Prostigmata were also recorded higher in the natural than that of the degraded study site as these groups were also predatory and detritus feeders and more preferred in organic and high humus layers (Sheals, 1956). yke and Loots (1967) reported Prostigmata as the dominant form among the total Acarina population in the study of 11 different South African soils. The least presence of Astigmata in different vertical layers and completely absent in degraded site may be attributed to its less tolerance ability, adaptation and limited food habit. Chitrapati (2002) also reported the presence of Astigmata only in upper most layer of the soil. Loots and Ryke (1966), Ryke and Loots (1967), Wood (1967) and Berg and Ryke (1968) mentioned that Astigmata were not important elements in the Acarina fauna of many soils and the roles of these mites in the soil community had been little studied. It has been observed that except for relative humidity, there was positive relationship between temporal variation of different physical factors and abundance of all groups of soil microarthropods in both natural and degraded forest ecosystem. However all chemical characteristics except soil potassium did not show any appreciable correlationship with soil microarthropods at different soil depth layers. High rain fall during rainy season increased the soil moisture, which together with high soil temperature made a congenial substratum for growth and development of soil microarthropds both in natural and degraded sites. It was generally believed that in tropical region the abundance of soil microarthropods was mainly regulated by soil moisture and rainfall
(Bhattacharya and Raychoudhuri, 1979 and Corpuz-Raros, 1980). The positive correlation
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1704
ISSN 2229-5518
between soil temperature and Soil micro arthropods such as Acarina in natural and degraded forest ecosystem from Imphal Valley has been reported by Chitrapati (2002). Reddy (1984) observed positive correlation with Acarina. Choudhuri and Banerjee (1977), Choudhuri and Pande (1979) and Sanyal (1982) reported the lack of significant correlation between pH and
Acarina.
Cryptostigmata (NF & DF) (a)
0 to 10 cm 10 to 20 cm
25 20 to 30 cm 0 to 10 cm
10 to 20 cm 20 to 30 cm
20
15
10
5
0
I15
J 20 to S30 cm
E0 to 10 cm R
Mesostigmata (NF & DF) (b)
0 to 10 cm 10 to 20 cm
10 to 20 cm 20 to 30 cm
10
5
0
Prostigmata (NF) (c)
0 to 10 cm 10 to 20 cm
5
4
3
2
1
0
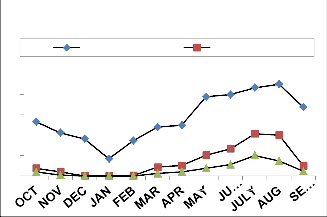
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1705
ISSN 2229-5518
Astigmata (NF) (d)
0 to 10 cm 10 to 20 cm
3
2
1
0
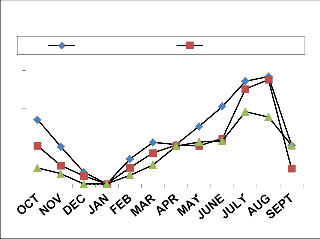
Total Acarina (NF & DF) (e)
0-10 10-20 20-30
40 0-10 10-20 20-30
30
10
20
0
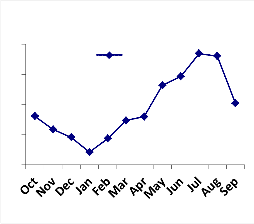
Fig. 3: Monthly vertical distribution of Acarina population in natural and degraded forest ecosystem (a)Cryptostigmata, (b)Mesostigmata, (c)Prostigmata (d)Astigmata
(e) Total Acarina ( Numbers x 102m-2)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1706
ISSN 2229-5518
0.819, p<0.001) and soil moisture (r = 0.856, p<0.001) in natural site as physical factors showed
to be positvely correlated in degraded site. But,no relationship was found between Acarina with relative humidity in both sites. The relationship with chemical factors was not found to be significant except soil potassium significant relationship with Acarina (r=-0.686, p<0.014) at 0-
10 cm, at 10-20 cm (r=-0.31, p<0.035) and at 20-30 cm (r=0.417,p<0.077) in natural site as also
degraded site.It was difficult to designate any single factor as the causative agent. However rainfall and soil moisture are the most important abiotic factors influencing the abundance.
4. Reference
Alfred, J.R.B., V.T. Darlong, S.J.S. Hattar, and D. Paul, 1991. Microarthropods and their conservation in some North- East Indian soil, In (Eds. G.K. Veeresh, D. Rajagopal and C.A.Virak Tamath): Advances in Management and conservation of soil fauna, Bangalore.
Aoki, J. I., 1967. Microhabitats of Oribatid mites on a forest floor, Bull, Nat. Sci., Mus. Tokyo,
10:133–138.
Badejo, M.A., T.O. Obilade and B.A. Oblubakin, 1997. Spatial distribution and abundance of mites and springtails under temperature and moisture regimes in a tropical rain floor. Tropical Ecology, 38 (1):31–38.
Badejo, M.A. and N.M. Van Straalen, 1993. Seasonal abundance of springtails in two contrasting environment. Biotropica, 25: 222–228.
Berg, R.A., Vanden and P.A.J.Ryke, 1968. A systematic ecological investigation of the acaro fauna of the forest floor in Magoobasllo of (South Africa) with special reference to the Mesostigmata. Rev. Biol., 6(1-2):157-234.
Bhandari, S.C. and L.L. Somani, 1994. In Ecology and Biology of soil organisms, Agrotech publishing
Academy, Uaipur.
Bhattacharya, T. and D.N. Raychoudhuri, 1979. Monthly variation in the density of soil microarthropods in relation to some climatic and edaphic factors. Entomon, 4(4):313-318.
Block, W.C., 1965. Distribution of soilmites on the Moor House National Nature Reserve, west moorland,
with notes on their numerical abundance. Pedobiologia, 5:244-251.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1707
ISSN 2229-5518
Chitrapati, C., 2002. Ecological study of soil microarthropods in the sub–tropical forest ecosystem at
Khonghapat, Manipur. Ph. D. Thesis, Manipur University.
Choudhuri, D.K. and S. Banerjee, 1977. Soil factors of soil oribatid mites under conditions of West
Bengal. The University of Burdwan Publication, 1-88.
Choudhuri, D.K. and T. Pande, 1979. High altitude soil animals and their relationship with soil factors.
With special reference to mites. Rev. Ecol. Soil, 16 (2):219–226.
Christiansen, K and P. Bellinger, 1980. The collembolan of North America: North of Rio Grande. Grinnel
College. Frinnel IA..
Corpuz-Raros, L.A., 1980. Philipine Oribatei (Acarna) V. Scheloribates Berlese and related genera
(Oribatulidae). J. Biol., Philipines 9:69-245.
Doulo, V., 2007. Population dynamics of microarthropods and effect of soil nutrients in natural and degraded forst ecosystem at Lumami, Nagaland. Ph. D. Thesis, submitted to Nagaland University.
Doulo, V. and L.N. Kakati,, 2009. Vertical distribution and seasonal variation of soil microarthropods in natural and degraded forest ecosystem at Lumami, Nagaland.. J. Soil Biol. Ecol., 29:126-138.
Hagvar, S.,1983. Collembola in Norwegian coniferous forest soil,1I- vertical distribution. Pedobiologia,
25:383-401.
Hattar, S.J.S. and J.R.B. Alfred and V.T. Darlong, 1998. Animal diversity in some managed and protected forests of North-East India with particular reference to soil fauna. (Eds by P.C. Kotwal and S. Banerjee).Pp.108–118, Agro Botanica, Bikaner.
Hazra, A.K., 1991. Effect of deforestation on the soil macro-microarthropod fauna of West Bengal, India, In: Advances in Management and conservation of soil fauna, (Eds, G.K. Veersh, D. Rajagopal and C.A. Viraktamath) Bangalore, pp. 399-411.
Karppinen, E., 1955. Ecological and transect survey studies on Faunistic Camisiids; Ann. Zool., Soc.
Vanamo 17:1-80.
Loots, G.C. and P.A.J. Ryke, 1966. A comparative quantitative study of the microarthropods in different types of pasture soils, Zool., Afr. 21: 157 – 197.
Luxton, M., 1966. Laboratory studies on salt marsh Acarina with notes on 203 their behaviour.
Acarologia, 8: 163-175.
Mitchell, M.J., 1977. Population dynamics of Oribatid mites (Acari : Cryptostigmata ) in an aspen woodland soil. Pedobiologia, 17:305-319.
Madge, D.S., 1965. Leaf fall and litter disappearance in a tropical forest, Pedobiologia, 5:273-288.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1708
ISSN 2229-5518
Narula, A., L.K.Vats and S.Handa., 1998. Collembolans and mites of eciduous forest stand, Indian Journal of foresty. 21(2): 147–149. Nguyen, T. T. and M.Q.Vu.1988.A population density and distribution of microarthropods (Microarthropoda: Acari, Collembola and others) in soils of tropical forest of plateau Tay Nguyen (CentralVietnam). USSR Journal of Ecology, 2:73–75.
Niijima, K.,1971.Seasonal changes in Collembolan population in a warm temperate forest of Japan.
Pedobiologia, 11:11-26.
Petersen, H., 1980. In: Soil Biology as related to land use practices, Dindal D.L.(ed). Proc. VIII Int. Soil
Zool., Colloq. Pp. 806-833.
Price, Price, D.W., 1973. Abundance and vertical distribution of microarthropods in the surface layers of a California pine forest soil. Hilgardia,. 42:121 – 174.
Rajagopal, D., 2011.Distributionof Mesostigmatid mites species in different habitats of Western Ghats. J.
Soil Biol.Ecol., 31(1&2):139-143.
Reddy, M.V., 1984. Ecology of Soil litter inhabiting arthropods, Indian Rev.Life Sci., 4:169–217.
Ryke, P.A.J. and G.C. Loots, 1967. The composition of the microarthropods fauna in South Africa soils. In Otto Graff and John E. Satchell, (eds). Progress in Soil Biology, Friedr. Vieweg and Sohr. Gmph, Brauchweig. 528-546.
Sanyal, A.K., 1982. Soil Oribatid mites and their relation with soil factors in West Bengal, J. Soil
Biol. Ecl., 2(1): 8 – 17.
Sanyal, A.K., 1995. Ecological studies of soil Mites (Acari) in India:A Review. In P.C.Mishra and N. Behra
(ed.) Advances in Ecology and Environmental Sciences, APH Publishing House, New Delhi, Pp.79-
96
Sarkar, S., 1991. Studies on microarthropod community in one undisturb habitat of Tripura with special reference to oribatid mites, In: Advances in management and Conservation of soil.
Seastedt, T.R., 1984. The role of microarthropods in decomposition and mineralization processes, Ann.
Rev. Entom., 29: 25 – 46.
Sheals, J.G., 1956. Soil population, studies I. The effects of cultivation and treatment with insecticides,
Bull, etc. Res. 47:803-22.
Singh, J. and Singh, U.R., 1975: An ecological study of soil microarthropoda upon soil and litter of a tropical deciduous forest of Varanasi (India). Trop. Ecol., 16:81-85.
Strong, J., 1967. Ecology of terrestrial arthropods at Palmer Station, Antarctic Peninsula., Anterctic Res.
Ser. 10:357-71.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 1709
ISSN 2229-5518
Wallwork, J.A., 1961. Some Oribatei from Ghana. V. Two members of the family Trhypochthoniidae, including a description of a new genus. Acarologia, 3: 232-241.
Wallwork, J.A., 1967. Acari, In “Soil Biology”, (Burges, N.A. and Raw, F. eds) Academic Press, London and
New York. PP. 363-395.
Wallwork, J.A, 1970. Ecology of soil animals, Mc graw- Hill publishing company limited. 283 pp. Wallwork, J.A, 1983. Oribatids in forest ecosystems, Annual Review of Entomology., 28:109-139.
Wardle, D.A. and K.E.Giller, 1996. The quest for a contemporary ecological dimension to soil biology- Discussion. Soil biology and biochemistry, 28:1549-1554.
Wood, T.G., 1967. Acari and Collembola of moorland soil from Yorkshire England. I. Description of the mites and their population. Oikkos, 18:102-117.
IJSER
IJSER © 2013 http://www.ijser.org